This post contains excerpts from Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Int J Chronic Dis. 2018 Mar 18;2018:9394060. doi: 10.1155/2018/9394060. eCollection 2018. Here are the excerpts:
Abstract
Patients with inflammatory conditions such as inflammatory bowel disease (IBD), chronic heart failure (CHF), and chronic kidney disease (CKD) have high rates of iron deficiency with adverse clinical consequences. Under normal circumstances, serum ferritin levels are a sensitive marker for iron status but ferritin is an acute-phase reactant that becomes elevated in response to inflammation, complicating the diagnosis. Proinflammatory cytokines also trigger an increase in hepcidin, which restricts uptake of dietary iron and promotes sequestration of iron by ferritin within storage sites. Patients with inflammatory conditions may thus have restricted availability of iron for erythropoiesis and other cell functions due to increased hepcidin expression, despite normal or high levels of serum ferritin. The standard threshold for iron deficiency (<30 μg/L) therefore does not apply and transferrin saturation (TSAT), a marker of iron availability, should also be assessed. A serum ferritin threshold of <100 μg/L or TSAT < 20% can be considered diagnostic for iron deficiency in CHF, CKD, and IBD. If serum ferritin is 100–300 μg/L, TSAT < 20% is required to confirm iron deficiency. Routine surveillance of serum ferritin and TSAT in these at-risk groups is advisable so that iron deficiency can be detected and managed.
Resources (2) and (3) below suggest that some cases of COPD are associated with significant chronic systemic inflammation. If so, then COPD would be an additional chronic disease to carefully look for iron deficiency in patients with non-specific symptoms that we don’t commonly associate with COPD.
1. Iron Deficiency in Inflammatory Diseases
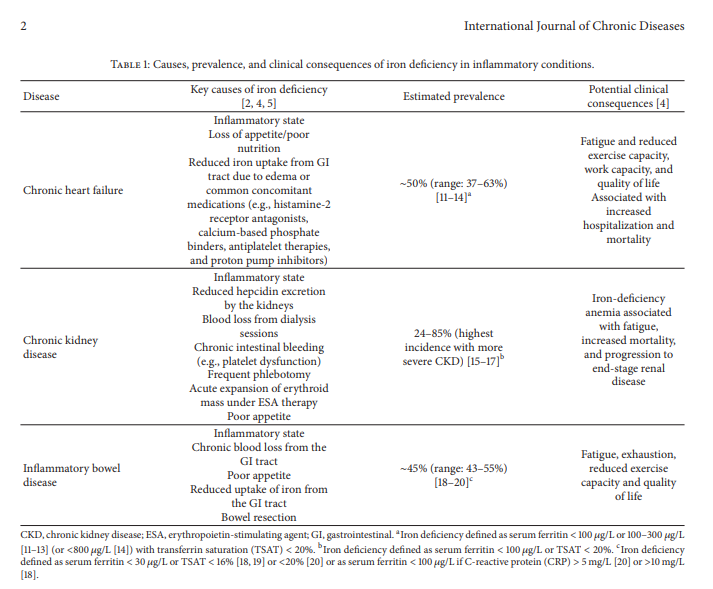
Conventionally, the most well-recognized risk groups for iron deficiency are the poorly nourished, those with high iron demands, such as pregnant women or adolescents, and individuals with chronic blood loss, for instance, from heavy uterine or gastrointestinal bleeding [5]. In addition, growing attention is now being paid to the iron status of patients with inflammatory conditions, which predispose them to iron deficiency [4, 6]. The most frequent of these are chronic heart failure (CHF), chronic kidney disease (CKD), and inflammatory bowel disease (IBD).
Estimates of iron deficiency in these groups have varied widely between studies due to differing definitions and diverse patient selection criteria. Overall, however, approximately 50% of patients with CHF, 24–85% of patients with CKD, and 45% of patients with IBD are iron-deficient (Table 1).
All too often, investigation and treatment of iron deficiency are only triggered by the onset of (iron deficiency) anemia, at which point iron deficiency has become severe enough to exhaust iron stores and restrict erythropoiesis. However, iron has multiple biochemical and physiological functions other than erythropoiesis [21] and iron deficiency exerts various adverse effects that may arise either before or after the onset of anemia. As well as being critical for erythropoiesis, iron is essential for the function of key enzymes in the mitochondrial electron transport system [22], which may explain the fatigue that can develop in nonanemic iron-deficient individuals. Iron deficiency has also been associated with poor immune function [23]. There is a clear need for systematic diagnosis and correction of iron deficiency in inflammatory conditions.
In normal circumstances, iron status can usually be assessed adequately by measuring serum levels of ferritin. In the presence of proinflammatory stimuli, however, the diagnosis of iron deficiency is more complex. Understanding the nature of serum ferritin and, particularly, how levels of serum ferritin are influenced by inflammation is key to successful diagnosis in this context.
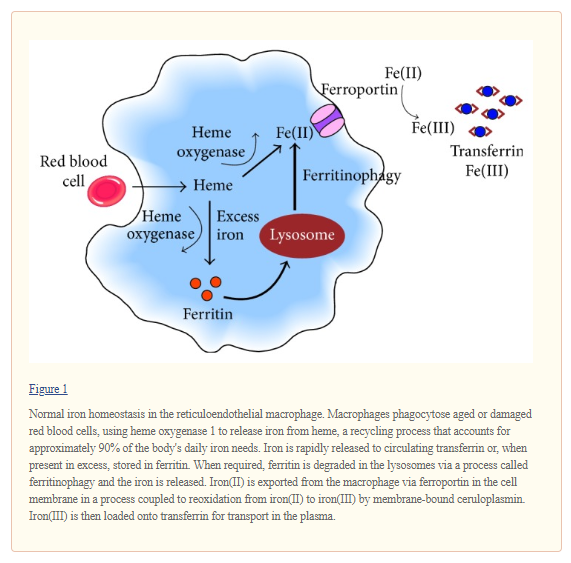
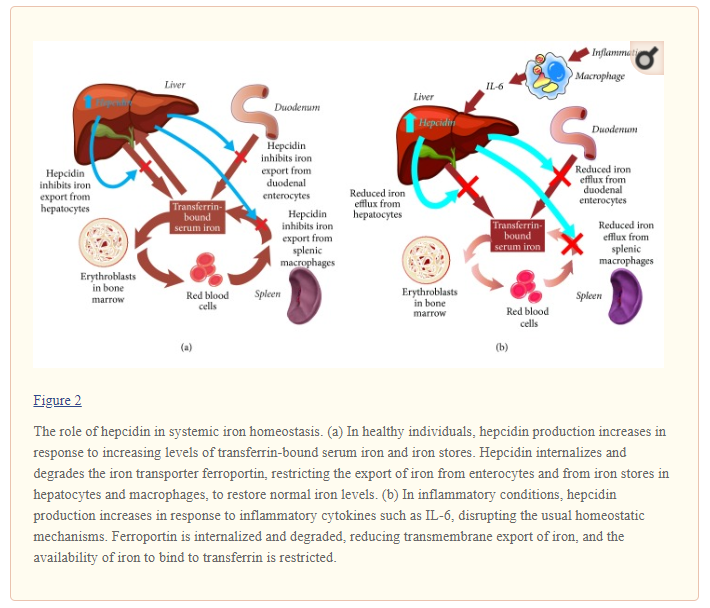
Systemic iron homeostasis is usually maintained in the face of fluctuating dietary iron intake and varying levels of demand by regulatory mechanisms coordinated by the hepatic hormone hepcidin. Hepcidin binds to and leads to internalization and degradation of the iron exporter ferroportin. This reduces the mobilization of iron into the circulation from enterocytes and from iron stores in hepatocytes and macrophages (Figure 2(a)) [41]. In healthy individuals, increasing levels of transferrin-bound iron and elevated iron stores stimulate hepcidin upregulation, which suppresses iron export and thus lowers circulating levels of iron [29, 41]. Conversely, hepcidin production is inhibited in the presence of declining levels of iron in the circulation and in tissues or in response to other stimuli such as hypoxia and intensified erythropoiesis after blood loss [29, 41]. In this situation, reduced levels of hepcidin stimulate increased iron acquisition and release by the enterocytes in the duodenum and efflux of ferritin-bound iron from storage sites to normalize iron availability and meet increased erythroid needs.
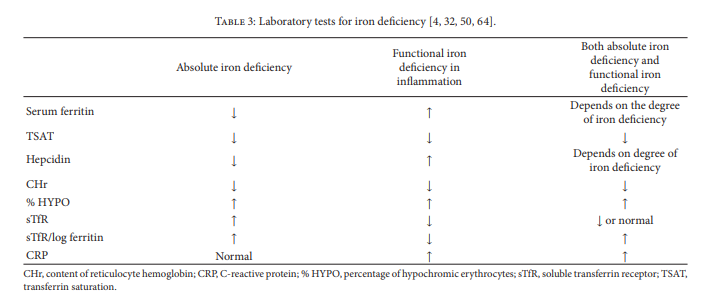
6. Diagnostic Thresholds for Serum Ferritin and TSAT in Inflammatory Conditions
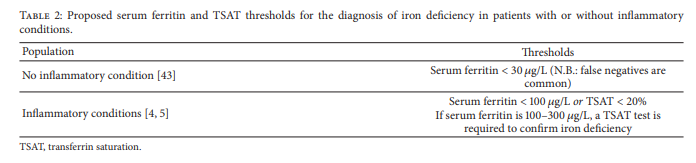
A simplified diagnostic approach in patients with CHF, CKD, or IBD recommends that iron deficiency be diagnosed based on the following cutoff values: serum ferritin < 100 μg/L or TSAT < 20%, and if serum ferritin is between 100 and 300 μg/L, a TSAT test is required to confirm iron deficiency [4] (Table 2). Hemoglobin levels may support the diagnosis of iron deficiency but do not need to be below normal to confirm the diagnosis [3]. This diagnostic approach has been used widely in recent large-scale prevalence studies of iron deficiency [11–13].
7. Other Diagnostic Tests for Iron Deficiency
Where infammation is present and serum ferritin with TSAT
testing is inconclusive, other tests may be necessary (Table 3).7.1. Hematological Markers. The percentage of hypochromic
erythrocytes (% HYPO) and the content of reticulocyte
hemoglobin (CHr or RetHb) are the most frequently used
hematological indices of iron status. [Review this section in the article for critical details.]7.2. Soluble Transferrin Receptor (sTfR) and the sTfR-Ferritin
Index. Soluble transferrin receptor (sTfR) is a truncated form
of transferrin receptor 1 (TfR). When TfR is not stabilized by
iron-laden transferrin, it is cleaved by a membrane protease
in erythroid cells, releasing sTfR. Levels of sTfR increase in
the presence of iron defciency and are reduced in patients
with iron overload [5, 32]. [This section of the article also needs to be reviewed for important details.]7.3. C-Reactive Protein. Assessing the severity of infammation
based on the level of high sensitivity CRP (hsCRP)
could theoretically be helpful in order to understand the
extent to which serum ferritin levels have risen as part of
the acute-phase response.However, there is currently no consensus on when to
include CRP in the diagnostic work-up for iron deficiency or what thresholds should be applied. CRP is not included
in guidelines for the evaluation of iron status in inflammatory
conditions [3]. In IBD, however, measurement of
CRP (with a threshold of 5 mg/L), or use of stool markers
such as calprotectin or lactoferrin, has been recommended
to confirm whether the disease is active or in remission, with
transabdominal ultrasound or endoscopy if required [4].Tis
allows serum ferritin results to be interpreted accordingly,
since levels are raised in active IBD [63].10. Conclusions
Iron deficiency often remains undiagnosed and untreated
in the context of inflammatory conditions [103]. It may
not be suspected because the typical symptoms, such as
fatigue, can be similar to those of the underlying disease.
Even in the absence of anemia, however, iron defciency can
negatively afect patients’ quality of life, and expert guidelines
in CHF, CKD, and IBD recognize that iron defciency should
be detected and managed [61–63, 104]. Routine laboratory
testing is advisable, with reassessment every 3 to 12 months or
in the event of disease progression [4]. Measurement of both
serum ferritin and TSAT offers a straightforward means to
identify the presence of iron deficiency in these at-risk groups
[4]. A diagnosis of iron deficiency can be made in these
conditions, regardless of whether anemia is present, if serum
ferritin is <100 micrograms/L or TSAT is <20%, using TSAT to confirm iron deficiency if serum ferritin is between 100 and 300 micrograms/L [4, 5]. This approach improves diagnostic sensitivity and allows prompt initiation of treatment. Iron replenishment can be achieved despite the presence of inflammation by use of intravenous iron therapies, as per expert guidelines [61–63, 104, 105]. The intravenous route bypasses the hepcidin induced blockade of oral iron uptake and release and avoids the problem of intolerance to oral iron [6, 106]. Clinical trials have shown intravenous iron to achieve iron repletion more rapidly and efficiently than oral iron, including studies in patients with inflammatory conditions [107–111]. Intravenous
iron should be avoided in case of potential infections.
With effective therapy available, surveillance of serum
ferritin and TSAT levels in these at-risk groups is prudent
so that iron defciency can be treated before progression to
symptomatic anemia or other complications. At the same
time, iron overload should be avoided, and markers to be
followed need to be established.
Resources:
(1) Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Int J Chronic Dis. 2018 Mar 18;2018:9394060. doi: 10.1155/2018/9394060. eCollection 2018.
(2) BURDEN OF DISEASE: Chronic Inflammation and Inflammatory Disease [Full Text PDF] from Phizer Value Of Medicines – A COLLECTION OF PAPERS DEMONSTRATING THE IMPACT OF MEDICINES ON PUBLIC HEALTH AND THE ECONOMY.
[From the above]
Chronic obstructive pulmonary disease (COPD) develops as a
significant and chronic inflammatory response to inhaled irritants.
Presumably, Phizer is suggesting that they have inflammatory immune suppressing medications that may be safer and more effective than inhaled or systemic corticosteroids which are the standard treatment for COPD.
(3) C-reactive protein level predicts mortality in COPD: a systematic review and meta-analysis [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Eur Respir Rev. 2017 Jan 31;26(143). pii: 160070. doi: 10.1183/16000617.0070-2016. Print 2017 Jan.