In this post I link to and excerpt from the outstanding IBCC chapter & cast – Toxic Alcohols [Link is to the podcast and show notes] from The Internet Book Of Critical Care [Link is to the Table Of Contents]. January 23, 2020 by Dr Josh Farkas:
CONTENTS
- Background
- Clinical features
- Diagnostic tests
- Treatment
- Evaluation summary
- Podcast
- Questions & discussion
- Pitfalls
- PDF of this chapter (or create customized PDF)
biochemistry
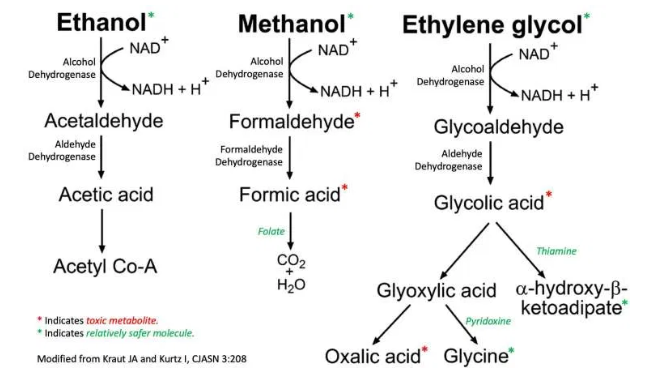
- Ethylene glycol and methanol themselves aren’t very toxic. However, alcohols are metabolized by alcohol dehydrogenase and aldehyde dehydrodgenase, producing toxic metabolites:
- Ethylene glycol ==> oxalic acid ==> calcium-oxalate precipitation in the kidney, causing renal failure.
- Methanol ==> formaldehyde and formic acid.
- Metabolic conversion explains biphasic clinical and laboratory findings:
- Clinical findings are initially due to alcohol; later on, they are due to toxic metabolites.
- Laboratory findings initially show elevated levels of toxic alcohol; later this disappears, and we see only acid metabolites.
- Treatment mirrors this.
- Early on: fomepizole*, to inhibit alcohol dehydrogenase (and thus prevent metabolite generation).
- *[Cost of fomepizole from drugs.com]
- *[Antidotes for Toxic-alcohol Poisoning from Medscape]
- Later on: once metabolites have been generated, dialysis may be needed to remove them.
mechanism of methanol toxicity:
- Mediated by formate, which is a mitochondrial toxin.
- Particularly affects retinas and basal ganglia.
mechanism of ethylene glycol toxicity:
- Glycolic acid
- Neurologic and cardiopulmonary manifestations.
- Oxalic acid
- Can cause precipitation of calcium-oxalate in kidneys and brain.
- Major driver of renal failure.
- Precipitation of calcium-oxalate may rarely cause symptomatic hypocalcemia.
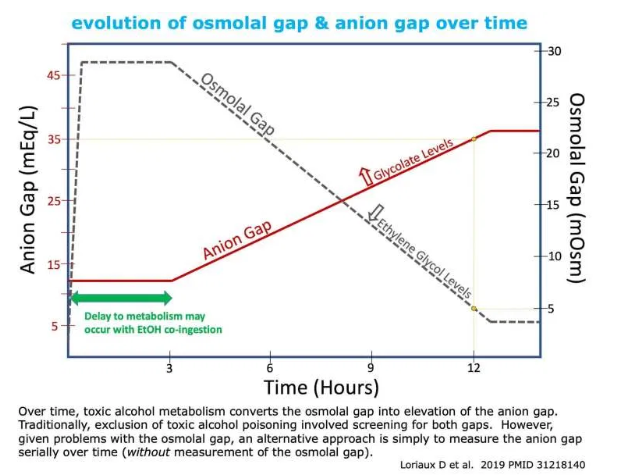
anion gap
Over time, the osmolal gap decreases while the anion gap increases. This may make the anion gap more useful than the osmolal gap. The anion gap may be strikingly high.
[Dr. Farkas states in the podcast and show notes that the osmolar gap is neither sensitive enough nor specific enough to rely on the osmolar gap.]
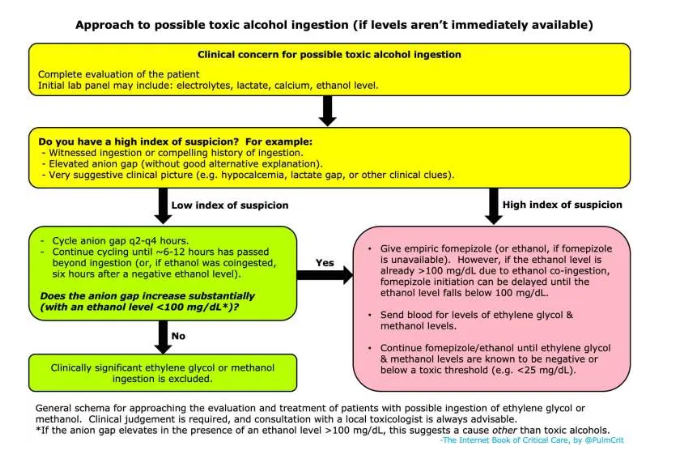
sensitivity
- Clinically significant poisoning with ethylene glycol or methanol will invariably cause anion gap elevation. The issue is when we can expect to see this elevation in anion gap.
- The relevant pharmacokinetics are as follows:
- Ethylene glycol and methanol are both readily absorbed from the gut (e.g. with peak serum levels occurring ~1 hour after ingestion).
- Ethylene glycol has a half-life of ~3-8 hours, whereas methanol has a half-life of ~2-3 hours. As parent alcohol levels fall, acidic metabolites rise.
- Metabolism may be halted by co-ingestion with ethanol (causing elevation of the anion gap to be delayed).
- Evidentiary support:
- Ethylene Glycol: Jolliff et al. retrospectively analyzed the published literature and found that an acidosis was invariably present by 4 hours after ingestion (provided the patient didn’t co-ingest ethanol)(abstract below).
- Methanol: Kostic et al. retrospectively analyzed all published literature and found that an acidosis was almost invariably present by >5 hours after ingestion (provided that the patient wasn’t treated early with fomepizole)(14677789).
- This suggests that, overall, serial measurement of anion gap over time should be extremely sensitive to toxic alcohol poisoning. This might be accomplished as follows:
- Anion gap could be measured at baseline and then repeated q2hr-q4hr.
- Exactly how long to follow the anion gap is unclear. Jolliff et al. below suggests that 4-6 hours should generally suffice for ethylene glycol. However, in the presence of ethanol co-ingestion, anion gap should arguably be cycled until 6 hours after the ethanol is metabolized. A recent review suggested cycling the anion gap for 12 hours (29427181).
- The intensity and duration of anion gap measurement could be adjusted based on clinical scenario and index of suspicion. For example, if the patient had a toxic ingestion >6 hours previously and ethanol levels are negative, then serial anion gap measurements might not be needed at all.