This post is first of a series I’ve made for my study notes on Cardiogenic Shock. This post contains links to and excerpts from Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Circulation. 2017 Oct 17;136(16):e232-e268.
The above article has been cited by 38 PubMed Central articles.
Here are excerpts [Note to myself, I’ve linked the title headings below directly to the title headings of the article-so I can easily read the full section]:
Cardiogenic shock is a high-acuity, potentially complex, and hemodynamically diverse state of end-organ hypoperfusion that is frequently associated with multisystem organ failure. Despite improving survival in recent years, patient morbidity and mortality remain high, and there are few evidence-based therapeutic interventions known to clearly improve patient outcomes. This scientific statement on cardiogenic shock summarizes the epidemiology, pathophysiology, causes, and outcomes of cardiogenic shock; reviews contemporary best medical, surgical, mechanical circulatory support, and palliative care practices; advocates for the development of regionalized systems of care; and outlines future research priorities.
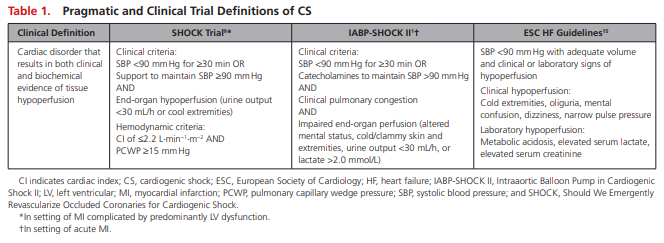
Acute cardiac hemodynamic instability may result from disorders that impair function of the myocardium, valves, conduction system, or pericardium, either in isolation or in combination. CS is pragmatically defined as a state in which ineffective cardiac output caused by a primary cardiac disorder results in both clinical and biochemical manifestations of inadequate tissue perfusion. The clinical presentation is typically characterized by persistent hypotension unresponsive to volume replacement and is accompanied by clinical features of end-organ hypoperfusion requiring intervention with pharmacological or mechanical support. Although not mandated, objective hemodynamic parameters for CS can help confirm the diagnosis and enable comparison across cohorts and clinical trials. Definitions in clinical practice guidelines and operationalized definitions used in the SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) and IABP-SHOCK II (Intraaortic Balloon Pump in Cardiogenic Shock II) trials are presented in Table 1.1,9,15
Historical Perspectives
Before the routine use of early revascularization, MI-associated CS had an in-hospital mortality exceeding 80%.
The first major breakthrough in CS treatment was achieved by the randomized SHOCK trial. Although an early invasive strategy coupled with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) did not reduce 30-day mortality (the primary outcome of the trial), a significant mortality reduction emerged at 6 and 12 months that persisted at longer-term follow-up.9,21,22 Subsequent registries confirmed the survival advantage of early revascularization.5,6,8
Further efforts to reduce CS mortality have been directed toward improvements in MCS devices. The largest randomized trial in patients with acute MI complicated by CS did not show a benefit with routine IABP placement in addition to revascularization.1 As a result, there has been a decrease in the use of IABPs in clinical practice and a downgrading in guideline recommendations.23,24 Recently, other percutaneous MCS devices have shown promise in the treatment of CS, but more data from randomized clinical trials are needed.25