In this post, I link to and excerpt from The Curbsiders‘ #321 Hypertension FAQ: Common Outpatient Cases with Dr. Jordy Cohen
FEBRUARY 14, 2022 By MATTHEW WATTO, MD
All that follows is from the above resource.
Master common hypertension scenarios in the clinic! Our guest Dr. Jordy Cohen (@jordy_bc) will lead us through the FAQs of outpatient hypertension management, including making a diagnosis of hypertension, managing blood pressure in patients with chronic kidney disease, working up refractory hypertension, and more.
Hypertension Pearls
- Given the difficulties with obtaining high-quality standardized blood pressure readings in the office, out-of-office home blood pressure measurement is recommended to confirm a diagnosis of hypertension. Patients should be instructed to acquire a validated blood pressure machine* and educated on the steps of taking their blood pressure. A minimum of three days of home blood pressure readings (two back-to-back readings every AM and two every PM) is sufficient.**
- *Patients should purchase a validated home blood pressure cuff. See the list of these cuffs at validatebp.org.
- **How can I measure my blood pressure at home? from The Centers For Disease Control and Prevention.
- For an initial blood pressure regimen, low-dose combination therapy is preferable to single agent therapy. A low-dose calcium channel blocker + ARB is a good starting regimen. Choose ARBs over ACE inhibitors due to their superior side effect profile. ARBs should be first-line in blood pressure management..
- 24 hour ambulatory blood pressure measurements are useful to diagnose white coat hypertension.
- For patients with chronic kidney disease, fear of a creatinine bump should not discourage providers from using ACEis/ARBs; on the contrary, these medications are protective in patients with kidney disease. Up to a 30% rise in creatinine with initiation of an ACEi/ARB is acceptable. Close monitoring of potassium is important, with a potassium threshold of 5.5.
- Diuretics are underutilized in patients with chronic kidney disease. Chlorthalidone is a promising agent in this population based on the CLICK trial.
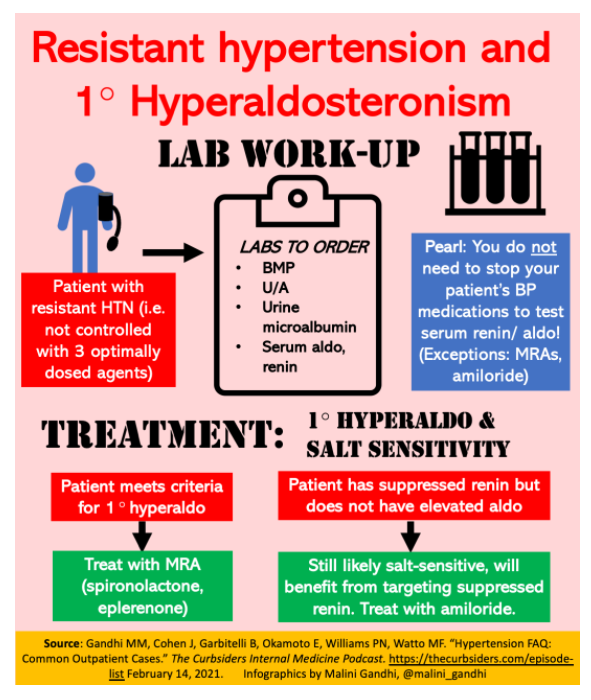
- In a patient with refractory hypertension, primary hyperaldosteronism should be on the differential. Testing serum renin and aldosterone does not require stopping your patient’s anti-hypertensive medications (with the exception of mineralocorticoid antagonists (MRA) and amiloride).
- Mineralocorticoid antagonists (spironolactone, eplerenone) are useful in treating patients with primary hyperaldosteronism.
- Salt-sensitive hypertension is likely if renin is suppressed even when aldosterone is not elevated. Thus, target renin suppression (ex: MRA or amiloride). Amiloride is a particularly good treatment option for this population.
Hypertension FAQ – Notes
Managing a new diagnosis of hypertension
How to confirm a new diagnosis of hypertension
To accurately make a diagnosis of hypertension, office readings need to be high quality, standardized measurements – which per Dr. Cohen are rarely done in practice. Patients need to have rested for several minutes beforehand, be sitting comfortably with their back supported, their feet on the floor, and their arm bare with the mid-arm at heart level, and be fitted with the correct cuff size (Unger et al, ISH guidelines, 2020). Yet Dr. Cohen notes that routine blood pressure readings in the clinic are frequently done with incorrect cuff sizes, over clothing, and with the patient sitting on the exam table (which leaves their back unsupported, their feet off the floor, and their arm dangling). A recent study comparing formal trial blood pressure measurements for patients in the SPRINT study with these same patients’ routine outpatient clinic blood pressure measurements found significant discordance between the two; while on average the routine clinic measurements were found to be slightly higher than the trial measurements, there was a high degree of variability, without a single common “correction factor” to convert between them (Drawz, 2020).
Given that standardized ideal office blood pressure measurements (which our research trials are based on) are so rarely actually achieved in practice, the USPSTF now recommends out-of-office home blood pressure checks to confirm a diagnosis of hypertension with Grade A evidence (USPSTF guidelines). Dr. Cohen notes that home blood pressures may actually be closer to approximating perfect “research quality” measurements since patients are often in a more ideal resting state when at home. Of course, obtaining reliable home measurements hinges on educating patients about the proper steps in taking their blood pressure. Dr. Cohen suggests the infographics on targetBP.org as good patient-friendly educational tools. Patients should be instructed to obtain a validated device, ideally, one listed on www.validateBP.org.
With regards to the number of blood pressure measurements needed to reliably estimate home blood pressure, Bello, 2018 indicated that a minimum of 3 days of morning and evening measurements was sufficient. Over those three days, Dr. Cohen recommends that patients obtain two consecutive readings in the morning (before their AM medications and before their morning coffee), and two consecutive readings in the evening about 12 hours later (before their PM medications.)
When to start anti-hypertensive medication for young, low-risk patients
For young, healthy patients without risk factors with blood pressures >130/80 but <140/90, ACC/AHA guidelines recommend counseling patients on lifestyle changes (including weight loss and low sodium / high potassium diets), without an indication for pharmacologic management; if their blood pressure is ≥140/90, pharmacologic intervention is recommended (Whelton et al, ACC/AHA guidelines, 2017.) However, Dr. Cohen notes that there isn’t much good data for young, low-risk patients with blood pressures between 130/80 and 140/90. In fact, many of these patients can’t even be entered into the ASCVD risk calculator for cardiovascular risk as they are too young! Dr. Cohen’s expert opinion is that she would prefer more stringent blood pressure control (<130/80). In her practice, she has an open, honest conversation with her young, low-risk patients with blood pressures in the 130-139/80-89 range, explaining that there isn’t good data for patients like them but that medication could potentially help with little risk.
Initial blood pressure regimen: Single agent vs. combination therapy
For patients that have borderline high blood pressures (eg 140/90), Dr. Cohen says that starting with monotherapy (for instance, low-dose amlodipine) may be appropriate. However, Dr. Cohen stresses that for patients with higher blood pressures (>150 systolic), she advocates for low-dose combination therapies as an up-front regimen. There is a growing body of literature in support of low-dose combination therapy as opposed to monotherapy for initial blood pressure management (Salaam et al, 2019).
Per Dr. Cohen, there are several benefits of low-dose combination therapy over single agents in the first-line setting:
- Starting a patient on multiple blood pressure agents with different mechanisms of action increases the likelihood that you will hit on the mechanism of their hypertension (ex: RAAS-driven hypertension vs sympathetic-drive hypertension vs. volume-driven hypertension). Additionally, for many patients, the mechanism of their hypertension may be multifactorial, and utilizing combination therapy can help address these complementary mechanisms of action.
- Starting with combination therapy allows you to start several medications at lower doses, rather than a higher dose of a single agent. Dr. Cohen emphasizes that maximum doses of blood pressure medications give you less “bang for your buck” in terms of blood pressure control, but significantly increase the risk of side effects.
What combination therapy to start with?
Dr. Cohen’s preferred combination therapy for initial treatment: low-dose calcium channel blocker (ex: 2.5 mg amlodipine) + ARB (ex: 10 mg olmesartan).
ACE inhibitors and ARBs
Is an ACE inhibitor or ARB better? Dr. Cohen prefers ARBs over ACE inhibitors given the superior side effect profile of ARBs, namely the lower risk of angioedema and cough (Chen, 2021). Given that ARBs are more affordable than they used to be, this is now more feasible.
Counseling patients on the teratogenicity of ACEis/ARBs and the need for birth control while on these medications is important prior to initiation.
Which ARB to choose? Dr. Cohen prefers olmesartan, given that it is longer-acting and thus minimizes blood pressure lability and can be dosed once per day. Losartan has a shorter half-life (4-6 hours) and needs to be given BID (though it does tend to be less expensive than olmesartan). Valsartan is generally a less potent ARB, and can be used if patients do not require as strong a medication for blood pressure control (with an option to switch to a more potent ARB later if needed.)
ACE inhibitors and race: Dr. Cohen favors ARBs as first-line in blood pressure management regardless of a patient’s race. Older guidelines indicated that Black patients should be started on calcium channels or thiazides and not ACEis/ARBs for initial blood pressure therapy. These recommendations were based on post-hoc analyses of the ALLHAT trial conducted in the 1990s, which suggested that ACEis/ARBs may be associated with a slightly increased risk of stroke in Black patients. However, Dr. Cohen notes that these studies are older with questionable elements, including issues relating to how the investigators defined race. Dr. Cohen encourages listeners to check out the work of her colleague Nwamaka Eneanya (@AmakaEMD) at University of Pennsylvania, who has done important work on the problems that arise from ascribing race to patients. Dr. Cohen stresses that this class of medications is very effective, and denying Black patients these agents simply based on a few questionable studies may be doing significant harm. She notes that Black patients have been shown to have higher rates of cough/angioedema from ACE inhibitors (Miller et al, 2008; Brown et al, 1996, Elliot 1996), and all patients are at risk for these side effects making ARBs an attractive first-line over ACE inhibitors for everyone.
Thiazides
Risk of hyperglycemia and hyperlipidemia: There is a reported risk of increased blood glucose and lipids with thiazides (Narzarzadeh et al 2021), though Dr. Cohen notes that the significance of this increase is controversial, given that these changes have not been found to impact the risk of cardiovascular events/mortality (Barzilay et al, 2006).
Dosing: For hydrochlorothiazide, Dr. Cohen recommends a starting dose of 25 mg (she notes that 12.5 mg, which is a commonly used starting dose, is actually much too low!) For chlorthalidone, Dr. Cohen recommends a starting dose of 12.5 mg daily. Unfortunately, the 25 mg pill is small and difficult to break in half, though she tells patients that it is okay if they can’t break it precisely in half given that chlorthalidone has such a long half life. Alternately, other providers have advocated for starting patients on one 25 mg pill of chlorthalidone every other day based on its long half life.
White coat hypertension
White coat hypertension describes the phenomenon of individuals who have elevated blood pressure when measured in the clinic but normal blood pressures when measured at home. White coat hypertension is especially prevalent in older women (Dolan, 2004).
Association between white coat hypertension and cardiovascular risk: Dr. Cohen led a meta-analysis investigating the association between white coat hypertension and cardiovascular risk (Cohen et al, 2019). Her group found that patients with white coat hypertension who were on a blood pressure medication did not have an elevated risk of adverse cardiovascular outcomes compared to normotensive individuals. On the other hand, patients with untreated white coat hypertension did have a significantly higher risk of cardiovascular events compared to normotensive patients. This increased risk of cardiovascular events in patients with untreated white coat hypertension was relatively small – about a 30% increased risk, compared to a 2-to-3 fold increased risk of cardiovascular events in patients with sustained hypertension – but it is still clinically important. Thus, per Dr. Cohen, white coat hypertension is not something to ignore.
How to assess for white coat hypertension: According to Dr. Cohen, patients who have elevated office blood pressure readings but who report normal blood pressure when they measure it at home are perfect candidates for 24 hour ambulatory blood pressure measuring. This strategy can help establish the diagnosis of white coat hypertension. It can also pick up other patterns of blood pressure that are important to be aware of for management – for instance, patients who have acceptable blood pressures when sitting down but significant hypertension otherwise, patients with highly labile blood pressure, or patients with severe nocturnal hypertension.
Masked hypertension: Masked hypertension is the exact opposite of white coat hypertension – it describes patients who have normal blood pressure in the office but elevated blood pressure at all other times. This condition is relatively common (Trudel, 2019) and underscores why it is so important to advocate for increased frequency of blood pressure measurement, according to Dr. Cohen.
Hypertension management in non-dialysis dependent chronic kidney disease
ACEis/ARBs for blood pressure control in CKD:
According to Dr. Cohen, we should not shy away from using ACEis/ARBs for blood pressure management in patients with chronic kidney disease! On the contrary, ACEis/ARBs have been shown to be protective in this patient population, reducing progression to end-stage kidney disease in patients with CKD and proteinuria (Jafar et al, 2001). ACEis/ARBs have also been shown to have significant cardiovascular benefits in this population (Mann et al, 2001). According to Dr. Cohen, while creatinine does bump with initiation of ACEis/ARBs, this rise does not hasten the decline to end-stage kidney disease; rather, it simply reflects a reduction in glomerular hyperfiltration that unmasks the patient’s true creatinine. In fact, Dr. Cohen considers a bump in creatinine to be a sign that the medication is doing its job!
Acceptable rise in creatinine for patients initiating ACEis/ARBs: A rise in creatinine of up to 30% within 4 weeks of initiating ACEis/ARBs is considered acceptable (Cheung et al, KDIGO guidelines, 2021). If the creatinine increases by >30%, this should prompt investigation of possible renal artery stenosis, likely bilateral.
Managing potassium in patients with CKD on ACEis/ARBs: Dr. Cohen considers a potassium of 5.5 to be the threshold at which she starts to become concerned in her patients with CKD on an ACEi/ARB. She notes that a potassium in the low 5s is usually not worrisome in this population, given that patients with CKD tend to have a higher potassium at baseline. According to Dr. Cohen, even if a patient’s potassium reaches the mid 5s on an ACEi/ARB, she may consider lowering the dose rather than stopping the ACEi/ARB entirely, and adding a diuretic if their blood pressure is still uncontrolled. Additionally, with the advent of safe, effective potassium binders such as sodium zirconium, it has become possible to keep patients on ACEis/ARBs for longer (though Dr. Cohen notes that adding on potassium binders does require a careful conversation about polypharmacy and consideration of patient goals).
Diuretics for blood pressure control in CKD:
Key point: Per Dr. Cohen, diuretics are “grossly underutilized” in patients with CKD.
Thiazides in CKD: Thiazides are promising agents for blood pressure control in CKD. The old heuristic that thiazide diuretics are no longer effective in advanced kidney disease because they can’t access the renal tubules has since been shown not to be the case. In particular, chlorthalidone is especially promising: the recent CLICK trial randomized patients with stage 4 kidney disease to chlorthalidone or placebo, and found that chlorthalidone achieved improved blood pressure control compared to placebo with good tolerability (Agarwal et al, 2021).
- Dosing: Dr. Cohen uses a starting chlorthalidone dose of 25 mg and titrates up from there.
Loop diuretics in CKD: According to Dr. Cohen, loop diuretics can be very useful for blood pressure management in patients with CKD, as patients tend to have hidden volume. Of the loop diuretics, Dr. Cohen prefers torsemide given its longer half-life, which can help reduce blood pressure lability as well as simplify medication regimens for patients (as it does not need to be taken BID).
- Dosing: Dr. Cohen uses a starting dose of 20 mg daily for torsemide, 1 mg BID for bumetanide, and 20-40 mg BID for furosemide.
Other medications for blood pressure control in CKD:
Calcium channel blockers in patients with CKD: Dihydropyridines like amlodipine and nifedipine do induce a minor increase in renal hyper-filatration, which can promote slight worsening of proteinuria (Nishida 2017). However, Dr. Cohen says these agents are not associated with worsened long-term renal outcomes in patients with CKD, and improve cardiovascular outcomes due to their effects on blood pressure control (Lin 2017). Non-dihydropyridines like diltiazem and verapamil are generally less potent anti-hypertensives. However, diltiazem is associated with a reduction in proteinuria (Gastri et al, 2004) and Dr. Cohen notes that it can be in your back pocket for proteinuric patients.
Hydralazine: Not a good option, per Dr. Cohen’s expert opinion – hydralazine promotes significant blood pressure lability, can worsen edema due to its potent vasodilatory effects, and overall tends to make patients feel poorly.
Beta-blockers: Also not a preferred option. In general, beta-blockers are not particularly effective anti-hypertensive agents, and are not go-to agents for blood pressure control unless a patient has a concurrent history of myocardial infarction or atrial fibrillation. Dr. Cohen does not favor utilizing beta-blockers until diuretic doses are maximized and volume is optimized. If beta-blockers are used, Dr. Cohen favors carvedilol due to its alpha-antagonist activity (though carvedilol does require BID dosing.)
Blood pressure goal in patients with CKD:
The KDIGO guidelines recommend a goal blood pressure for patients with CKD of <120 systolic (KDIGO guidelines, 2021.) However, Dr. Cohen notes that it can be very difficult to meet this target, particularly in patients with CKD.
Refractory hypertension
Diagnosing refractory hypertension
Definition: To qualify as having refractory hypertension, a patient must be on three optimally dose anti-hypertensive medications without achieving adequate blood pressure control (make sure you trust your blood pressure readings!) There is controversy over whether one of these three medications needs to be a diuretic.
Key questions to consider in a patient with refractory hypertension:
- Does the patient have untreated sleep apnea?
- Does the patient have CKD?
- Could the patient have primary hyperaldosteronism? (See work-up detailed below).
Lab work-up for refractory hypertension
Important lab tests to send when investigating refractory hypertension:
- Basic metabolic panel
- Urinalysis
- Urine microalbumin
- Serum aldosterone and renin
If there is a high level of suspicion for pheochromocytoma or Cushing’s (e.g. corresponding signs / symptoms), Dr. Cohen will also check plasma metanephrines* or 24-hour urine cortisol.
*See Pheochromocytoma Workup
Updated: Aug 20, 2021 from emedicine.medscape.com
Author: Michael A Blake, MBBCh, MRCPI, FRCR
Key point: testing serum renin and aldosterone does NOT require stopping all of your patient’s anti-hypertensive medications! Dr. Cohen stresses that if a patient is in a true state of aldosterone excess, renin will be suppressed, and it is very difficult to overcome this suppressed renin with medications. Per Dr. Cohen, stopping anti-hypertensive medications for these lab tests is unnecessary and unsafe. Most anti-hypertensives may be continued during screening for primary aldosteronism by determination of renin and aldosterone concentration (Seifarth 2002). The only exceptions to this rule are 1) mineralocorticoid antagonists (which need to be stopped at least one month prior to testing renin and aldosterone), and 2) high-dose amiloride.
Interpreting aldosterone level: An aldosterone level >15 with a suppressed renin is typically considered primary hyperaldosteronism (modified from the classic aldosterone cut-off of >20.) Dr. Cohen also indicates that if a patient has borderline aldosterone results that don’t quite meet the cut-off but she has a high level of suspicion for aldosterone excess, she will re-check the aldosterone/renin levels in a few months, as the assays are not always reliable. Additionally, even if a patient does not have particularly elevated aldosterone levels but they do have suppressed renin, Dr. Cohen still considers them to be an individual who is likely salt-sensitive and thus likely to benefit from therapy targeting renin suppression.
Treatment for primary hyperaldosteronism
Mineralocorticoid receptor antagonists: Spironolactone and eplerenone can both be used for patients with primary hyperaldosteronism. Before starting spironolactone, male patients should be counseled on side effects of gynecomastia and erectile dysfunction. For both medications, be sure to monitor potassium and sodium within the first few weeks of starting.
- Dosing: For spironolactone, Dr. Cohen tends to start with 25 mg for patients with low potassium, and 12.5 mg for patients with higher potassium. For eplerenone, Dr. Cohen uses a starting dose of 25 mg.
Amiloride: Per Dr. Cohen, amiloride is an especially good option for patients with suppressed renin but not particularly high aldosterone, i.e. patients who do not meet aldosterone criteria for primary hyperaldosteronism, but are likely still salt-sensitive. Dr. Cohen considers these patients to have a “Liddle-like” syndrome, which is treated with amiloride (Monticone, 2018.) Watch out for nausea and lightheadedness during the first few days after initiation.
- Dosing: Dr. Cohen uses a starting amiloride dose of 2.5mg or 5 mg daily. If additional blood pressure control is needed, the dose can be increased to 5 mg BID. Beyond that, if patients remain hypertensive, eplerenone can be added on.