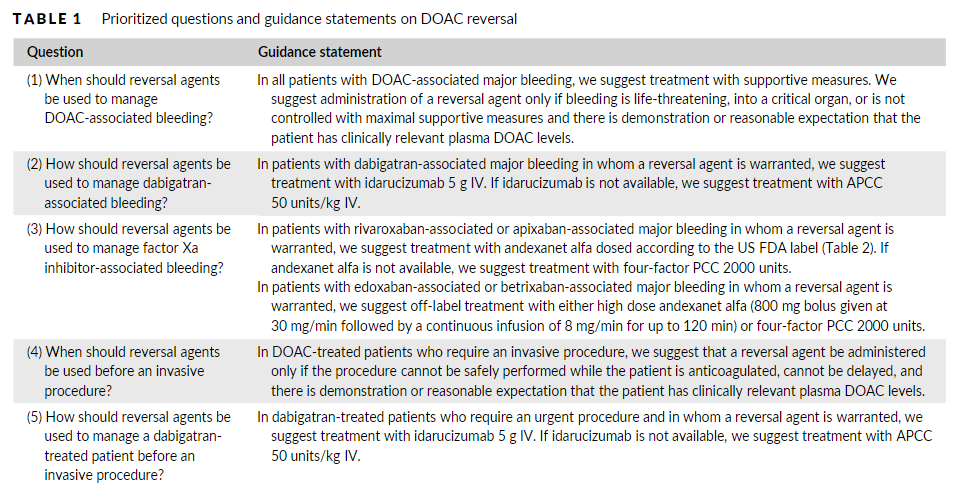
In this post I link to and excerpt from Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Am J Hematol. 2019 Jun;94(6):697-709.
Here are excerpts:
Abstract:
Two specific reversal agents for direct oral anticoagulants (DOACs) have been approved in the United States: idarucizumab for dabigatran reversal and andexanet alfa for apixaban and rivaroxaban reversal. Non-specific prohemostatic agents such as prothrombin complex concentrate (PCC) and activated PCC have also been used for DOAC reversal. The goal of this document is to provide comprehensive guidance from the Anticoagulation Forum, a North American organization of anticoagulation providers, regarding use of DOAC reversal agents. We discuss indications for reversal, provide guidance on how the individual reversal agents should be administered, and offer suggestions for stewardship at the health system level.
1 | INTRODUCTION
The direct oral anticoagulants (DOACs) comprise the direct thrombin inhibitor, dabigatran, and the direct factor Xa inhibitors, apixaban, betrixaban, edoxaban, and rivaroxaban. Collectively, these agents have been approved by the United States Food and Drug Administration(US FDA) for prevention of stroke and systemic embolism in non-valvularatrial fibrillation, prevention and treatment of venous thromboembolism(VTE), and secondary prevention of arterial ischemic events in patients with chronic coronary or peripheral artery disease.1–5
Two key advantages of the DOACs compared with VKAs are a reduced incidence of major bleeding and simplified perioperative management. 9,10 Nevertheless, patients taking DOACs may present with serious bleeding or need for an urgent unplanned procedure.Major bleeding was reported in 2.1% to 3.6% of patients randomized to treatment with a DOAC in phase III clinical trials. Two DOACs, along with warfarin, are among the top tendrugs contributing to emergency department visits in the US.9,11,12
Two specific DOAC reversal agents have been approved by the US FDA: idarucizumab (Praxbind, Boehringer Ingelheim) for reversal of dabigatran and andexanet alfa [coagulation factor Xa (recombinant) inactivated-zhzo; Andexxa, Portola Pharmaceuticals] for reversal of apixaban and rivaroxaban.13,1Non-specific prohemostatic agents have also been used off-label for DOAC reversal including prothrombin complex concentrate (PCC) (multiple brands) and activated prothrombincomplex concentrate (APCC) [FEIBA (Anti-Inhibitor Coagulant Complex), Takeda Pharmaceutical Company]. 15,16
Various factors complicate the use of these agents in clinical practice including availability, potential risk of thrombosis, cost, preparation, and a lack of data on the comparative effectiveness of different reversal strategies. Moreover, US FDA-approved reversal agents are not indicated for use with all DOACsor in all clinical scenarios where reversal may be considered.13,14