In this post I link to and excerpt from Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Am Heart Assoc. 2020 Jul 7; 9(13): e017559.
Here are excerpts:
ABSTRACT: Direct oral anticoagulants (DOACs) have quickly become attractive alternatives to the long-standing standard of care in anticoagulation, vitamin K antagonist. DOACs are indicated for prevention and treatment of several cardiovascular conditions. Since the first approval in 2010, DOACs have emerged as leading therapeutic alternatives that provide both clinicians and patients with more effective, safe, and convenient treatment options in thromboembolic settings. With the expanding
role of DOACs, clinicians are faced with increasingly complex decisions relating to appropriate agent, duration of treatment, and use in special populations. This review will provide an overview of DOACs and act as a practical reference for clinicians
to optimize DOAC use among common challenging scenarios. Topics addressed include (1) appropriate indications; (2) use in patients with specific comorbidities; (3) monitoring parameters; (4) transitioning between anticoagulant regimens; (5) major drug interactions; and (6) cost considerations.Key Words: anticoagulation ■ oral direct thrombin inhibitor ■ oral factor Xa inhibitors ■pharmacotherapy
Direct oral anticoagulants (DOACs)—dabigatran
(Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), edoxaban (Savaysa), and betrixaban (Bevyxxa) are anticoagulation pharmacotherapy used for the prevention of thrombosis in several cardiovascular contexts.1 DOACs are categorized into 2 main classes: oral direct
factor Xa inhibitors (ie, rivaroxaban, apixaban, edoxaban, and betrixaban) and direct thrombin inhibitors (ie, dabigatran).DOACs are relatively new agents demonstrating superiority or noninferiority to prior standards of
care, anticoagulation with vitamin K antagonists (VKA; ie, warfarin), or low-molecular-weight heparins (LMWHs), in reducing risk of thromboembolic complications with similar or reduced bleeding risk.2–5The purpose of this review is to be a practical reference or algorithm for the busy clinician to navigate key aspects of effective DOAC prescribing, with an emphasis on addressing key situations where clinical uncertainty exists. This review will provide recommendations to address special
clinical situations to include indications, use in specific comorbidities, monitoring parameters, transitioning to or off of therapy, drug interactions, and cost.
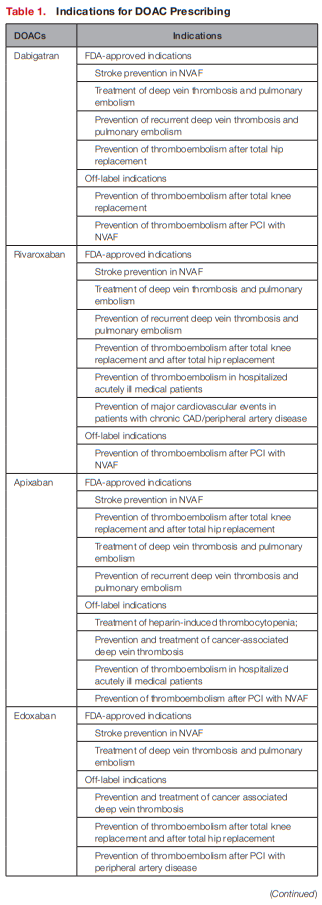
In general, FDA-approved indications for each of the
DOACs are comparable (see Table 1).
Dabigatran, rivaroxaban, apixaban, and edoxaban are approved for the lowering the risk of stroke and embolism in NVAF as well as deep vein thrombosis and pulmonary embolism treatment/prophylaxis.8–11
Unique indications include betrixaban for prophylaxis of venous thromboembolism (VTE) in hospitalized patients for an acute medical illness, and rivaroxaban in combination with aspirin to reduce major cardiovascular events in patients with
chronic coronary artery disease (CAD) or peripheral
artery disease.9,12However, there is still uncertainty in understanding safe and effective use of DOACs in the setting of patients with cardiovascular comorbidities,
specifically atrial fibrillation (AF) with recent percutaneous coronary intervention (PCI), AF with concomitant artificial heart valves, stable atherosclerotic cardiovascular disease (ASCVD), and cancer-associated thromboembolism.[The following sections aim] to clarify the use of
DOACs within these patient subgroups.AF and PCI
[See PDF pp 2+3 for details]
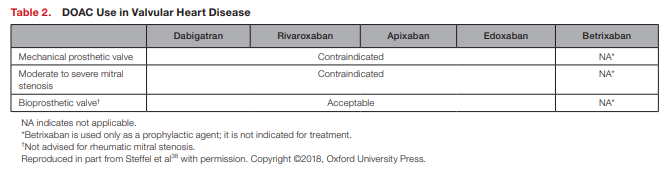
AF and Artificial Heart Valves
[See PDF pp 3+4 for details]
Stable Atherosclerotic Cardiovascular
DiseaseAspirin use for secondary prevention of ASCVD is well established and is widely recommended by major clinical guidelines for indefinite use.39 In this setting, major ASCVD events are reduced ≈20% to 30%, but major bleeding increased ≈1.4 to 1.6-fold.40,41 In an effort to expand upon the ASCVD risk reduction witnessed with aspirin monotherapy, prior investigations evaluated more potent antiplatelets (ie, clopidogrel, ticagrelor, and vorapaxar) and VKA therapy as alternatives
to aspirin and as add-on therapy with aspirin.42The recurring theme with the use of these strategies was a slight reduction in ASCVD events but at the expense of increased bleeding episodes and no effect on mortality. After promising results were hinted at with combining antiplatelet therapy with low-dose rivaroxaban in patients with acute coronary syndrome undergoing
PCI, investigations into use of this strategy for chronic ASCVD were sought.43The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) study demonstrated that rivaroxaban plus aspirin had
significant secondary prevention benefits for patients with ASCVD (ie, CAD and peripheral artery disease), which led to FDA approval for this indication in 2018.44Patients were randomized to 3 treatment arms: rivaroxaban 2.5 mg twice daily with aspirin 100 mg daily; rivaroxaban 5 mg twice daily alone; or aspirin 100 mg daily alone.44 This is a drastic dose reduction for rivaroxaban from the recommended 20 mg daily dose approved for patients with NVAF and VTE, but the rationale for low doses was provided by previous trials.42
The benefits of therapy were slightly offset by the increased risk in major bleeding by 1.70-fold (P<0.001). Nonetheless, the reduced rivaroxaban dose in combination with aspirin did not increase fatal or critical organ bleeding, and the net clinical benefit still favored the combination therapy.44
Overall, the COMPASS trial demonstrated significant benefit in reducing major cardiovascular events, a composite of cardiovascular death, stroke, and myocardial infarction by 24% (P<0.001), and major adverse limb events (including amputations) by 46% (P=0.0037) with low doses of rivaroxaban in combination with aspirin.44,45 This novel indication for rivaroxaban provides an important new antithrombotic therapy regimen for a large group of high-risk patients with established ASCVD and another pathway for reducing residual atherosclerotic risk.
A common clinical conundrum occurs in the setting of stable ASCVD and concomitant AF: What is the most effective long-term antithrombotic regimen? This question was addressed in AFIRE (Antithrombotic Therapy for Atrial Fibrillation With
Stable Coronary Disease).46 The trial randomized
2236 Japanese patients with AF who had undergone
PCI or coronary artery bypass grafting >1 year earlier or angiographically confirmed CAD not requiring revascularization to rivaroxaban 15 mg daily (10 mg daily with creatinine clearance [CrCl] 15–49 mL/min) or combination therapy consisting of rivaroxaban plus an antiplatelet (aspirin or P2Y12 inhibitor). The doses of rivaroxaban were lower than the FDA approved doses (20 mg with normal and 15 mg with impaired renal function) because of pharmacokinetic modeling revealing that Japanese patients achieve similar plasma levels of rivaroxaban compared with 20 mg in Caucasian patients.47The AFIRE trial was stopped early because of increased mortality in the combination therapy group (1.85% versus 3.37% per patient year; HR, 0.55; 95% CI, 0.38–0.81). The rivaroxaban monotherapy arm was noninferior in cardiovascular efficacy measures (P<0.001) and superior in reducing major bleeding (P=0.01) compared with the combination therapy group.
Thus, the results of the AFIRE study extended the concept of less intense antithrombotic for optimal risk-benefit established in the acute-care setting from PIONEER AF-PCI,16 RE-DUAL PCI,17 and AUGUSTUS,18 to the time period beyond a year from diagnosis and intervention.
Cancer-Associated Thromboembolism
[What follows is only an excerpt from this section of the paper. Please see the PDF p 5 for the full details.]
The ADAM VTE (Apixaban, Dalteparin, in
Active Cancer Associated Venous Thromboembolism) trial was the first trial for apixaban to support active treatment of cancer-associated VTE.53 Apixaban dosed at 10 mg twice daily×7 days, then 5 mg twice daily thereafter, was compared with dalteparin (dosed as above) over 6 months. Apixaban was associated with a noninferiority with regard to the primary end point of major bleeding (0% versus 2.1%; P=0.9956) and a robust 74% relative risk reduction in recurrent VTE (P=0.0182).Similar results were recently published in the Caravaggio (Apixaban for the Treatment of Venous Thromboembolism Associated With Cancer) trial, demonstrating noninferiority with respect to recurrent VTE (P<0.001) and comparable
major bleeding (P=0.60).54 Thus, the current landscape for treatment of VTE in the cancer population seems to support apixaban over the current standard of care with LMWH and other DOACs, establishing the ideal balance of thrombosis prevention while not inducing excess bleeding.In response, major guidelines now endorse the use of DOAC in cancer-associated VTE treatment.55,56 Trials investigating the use of DOACs for the prevention of VTE in patients with cancer have
also been conducted and support the use of these
agents in patients with cancer undergoing ambulatory chemotherapy with intermediate-high VTE risk and low bleeding risk.48,57,58As current practice heavily favors LMWH and VKAs, these novel and compelling trials supporting the use of DOACs will likely serve to ignite a paradigm shift in practice standards among this growing population of complex patients.
COMORBIDITIES AFFECTING DOAC
PHARMACOKINETICSPatient-specific comorbidities, particularly ones that
affect systemic exposure of the medication, need to
be taken into account when managing patients on
DOAC therapy. Comorbidities may alter the DOAC
elimination rate, therefore increasing the risk of a
thromboembolic or bleeding events. Of the many
factors that need to be considered, we will address
the 3 most pertinent patient comorbidities: renal
impairment, hepatic impairment, and extreme body
weights.Renal Insufficiency
Patients with chronic kidney disease (CKD) have an increased risk for thromboembolic and bleeding events.
All DOAC therapies are eliminated by the kidneys to varying degrees, and alterations in renal clearance must be taken into account when dosing these agents. Dabigatran is the most renally eliminated,
accountable for 80% of its clearance pathway, followed by edoxaban, rivaroxaban, apixaban, and betrixaban: 50%, 35%, 27%, and 11%, respectively.38,59Any clinical decisions on how to treat patients with DOACs requires the assessment of patient renal function, which should be monitored frequently, at least annually or more frequently if a patient has additional comorbidities and risk factors.
Renal dose adjustments include a decreased
dose or decreased frequency of the regimen, and for
most DOACs are based on the Cockcroft-Gault CrCl*
equation to estimate renal function.*Link is to the equation on mdcalc.com.
Phase III trials involving DOACs have excluded patients with severe renal dysfunction (CrCl <30 mL/min) or dialysis.22,23,25
Knowledge of when and how to renally dose adjust is imperative to appropriate DOAC prescribing.
Inappropriate dosing of DOAC carries grim consequences, with increased risk for thrombotic and bleeding complications resulting from subtherapeutic and supratherapeutic dosing, respectively.72
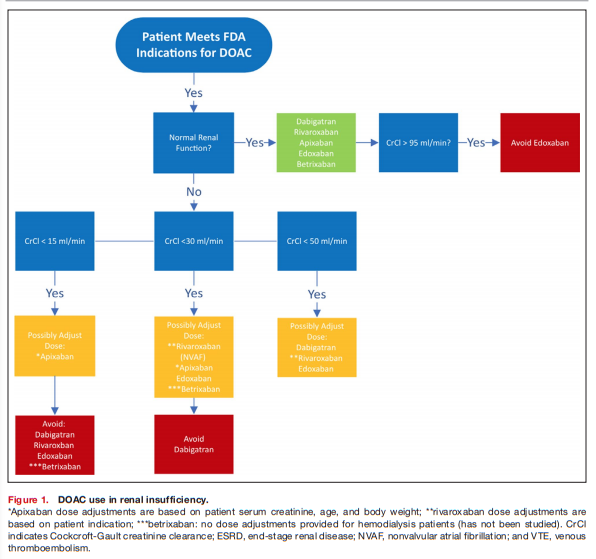
In summary, DOACs are safe and effective in patients with moderate CKD (CrCl 30–50 mL/min).
Dabigatran, rivaroxaban, and edoxaban should undergo dose adjustment for renal impairment and
should be avoided for severe renal impairment (CrCl
<30 mL/min). Edoxaban should be avoided in those
with normal renal function (>95 mL/min). Apixaban and betrixaban undergo the least amount of renal elimination and may be the DOACs of choice in severe renal impairment. See Figure 1 for suggested DOAC use based on patient renal function.
Hepatic Impairment
Similar to other disease states noted above, patients with hepatic impairment are at increased risk
of bleeding complications and thrombotic events.73
Alterations in hepatic function affects DOAC biotransformation to varying extents. Apixaban is the most reliant on hepatic metabolism for drug elimination, accounting for 75% of its elimination pathway, followed by rivaroxaban, edoxaban, dabigatran, and betrixaban: 65%, 50%, 20%, and up to 18%, respectively.12,73 Rivaroxaban and apixaban require cytochrome P450 (CYP) enzymes for metabolism, while dabigatran and edoxaban require no to minimal CYP metabolism.73 There was minimal hepatic elimination of betrixaban, and it is not metabolized by CYP enzymes, nor does it induce or inhibit CYP activity.67As there is no great monitoring parameter to assess
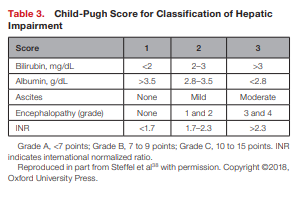
for safety, patients with hepatic dysfunction may not be ideal candidates for these agents.Restrictions for the use of DOACs in patients with hepatic impairment are based on the Child-Pugh classification system and exclusion criteria applied in pivotal trials (see Table 3).38,73,74
The Child-Pugh score uses the presence of clinical and biochemical abnormalities to assess the severity of the hepatic dysfunction.
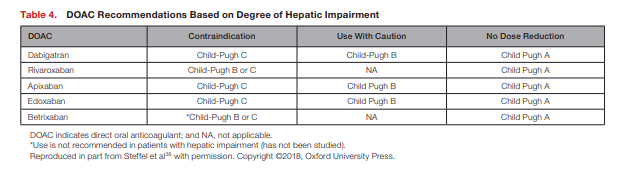
All DOACs are contraindicated in patients with severe hepatic disease in which warfarin is the only recommended anticoagulant in this patient population.38
Dabigatran, apixaban, and edoxaban are viable options in patients with moderate hepatic impairment and do not require dose adjustments.38 All DOACs could be considered in patients with mild hepatic impairment without any dose adjustments.
With the lack of information, the optimal anticoagulation strategy for this patient population remains unclear and it is recommended to obtain blood tests to evaluate hepatic function and coagulation parameters before initiating and periodically throughout DOAC therapy.
Summarized in Table 4 are recommendations for patients with hepatic impairment based on their Child-Pugh classification.
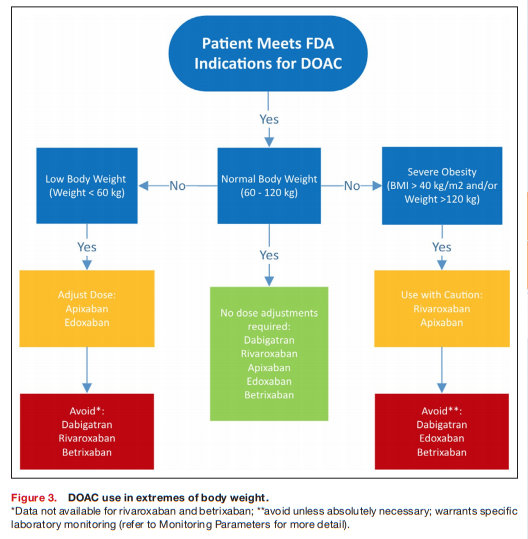
Extreme Body Weights
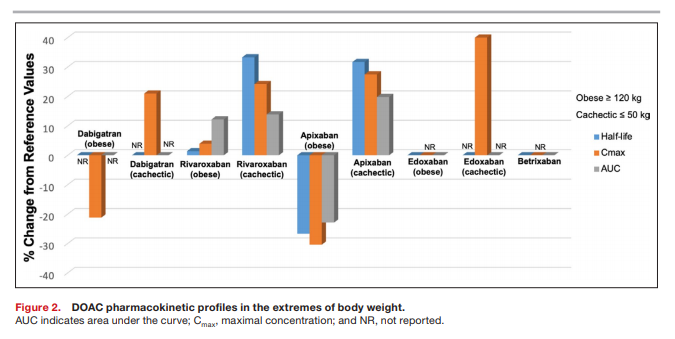
Optimal anticoagulant agents and dosing strategy for patients with extreme body weights have not been established for DOACs. Concerns have arisen with the use of DOACs in patients with extremes of body weight attributable to physiological changes that affect clearance of the medication and may lead to adverse outcomes, as well as limited data to guide prescribers. Fixed drug doses may lead to decreased drug exposures in obese patients and increased drug exposures in underweight patients based on drug pharmacokinetic changes (see Figure 2).75–80
An analysis of trials conducted by the International Society on Thrombosis and Haemostasis suggests that DOACs are safe in patient ≤120 kg (body mass index ≤40 kg/ m2) at standard doses and are not recommended in patients >120 kg (body mass index >40 kg/m2).82 [See the rest of the section on PDF p 8 for more (difficult to follow) details.]
Among low-body-weight patients (<60 kg), measuring renal function plays an important role in assessing use of DOACs, as renal function is commonly overestimated in this population because of lower muscle mass.
In addition, low-body-weight patients commonly present with comorbid conditions (ie, elderly age, frailty, and renal impairment) that predispose to
adverse outcomes.38DOACs with recommended dose reductions based on low body weight (≤60 kg) include apixaban (in addition to age and renal function) and edoxaban—both as a result of pharmacokinetic evaluation demonstrating increased systemic exposure in this population (see Figure 2). [See the rest of this section on PDF p9 for the again, difficult to follow, discussion.]
Overall, pharmacokinetic data suggest extreme body weights affect DOAC disposition, but this has not been overtly confirmed in limited trial data. Recommendations for DOAC use in patients of extreme body weight are listed in Figure 3.38,77,82
Monitoring Parameters
There are no FDA-approved method to monitor the
anticoagulant effect of DOACs.Quantitative measures exist such as anti–factor
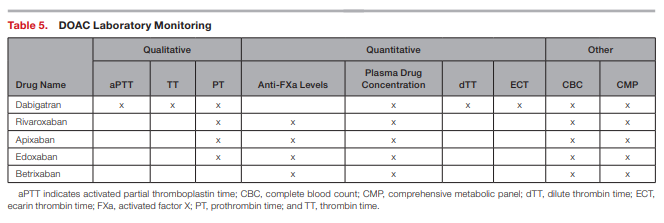
Xa levels, plasma drug concentrations, dilute thrombin time, and ecarin thrombin time to directly assess anticoagulation effects.90,91 However, quantitative tests do not have an established clinical role, since standardized therapeutic ranges have not been established and quantitative test results have not been correlated with clinical outcomes.Other general monitoring parameters include signs and symptoms of bleeding, complete blood count, and a comprehensive metabolic panel specifically evaluating liver function tests, albumin, total bilirubin, and serum creatinine (see Table 5).
These monitoring parameters should be performed in all patients before initiation of a DOAC and routinely monitored 1 to 3 months after initiation and then every 6 to 12 months thereafter, or more frequent follow-ups based on patient-specific characteristics is recommended in provider plans.
In addition to laboratory monitoring parameters, a comprehensive DOAC monitoring approach should include the following items assessed at each follow-up:
1. Current health status.
2. Adherence and cost.
3. Bleeding/thromboembolic events.
4. Adverse effects.
5. Complete medication review for drug interactions.
6. Reassessment of appropriateness of therapy.
7. Repeat warranted laboratory parameters.
8. Continuing follow-up appointments for patients.ANTICOAGULANT TRANSITIONING
As with any high-risk medication such as anticoagulants, clinical situations may arise that require transitioning to or off of DOAC therapy. Two common transition scenarios include (1) switching between anticoagulants and (2) periprocedural management.
Switching Between Anticoagulants
1. VKA to DOAC
2. DOAC to VKA
3. DOAC to DOAC
4. DOAC to parenteral anticoagulant
5. Parenteral anticoagulant to DOACFor all scenarios, assessment of each drug’s pharmacokinetic profile (ie, half-life), pharmacodynamics profile (INR for VKA or activated partial thromboplastin time
for unfractionated heparin, etc), as well as patients’ renal function, must be taken into account. The ultimate goal is to limit interruption of the therapeutic anticoagulation during the transition to minimize the risk of atherothrombosis. In certain cases, this requires overlap of the 2 anticoagulants. See Table 6.92
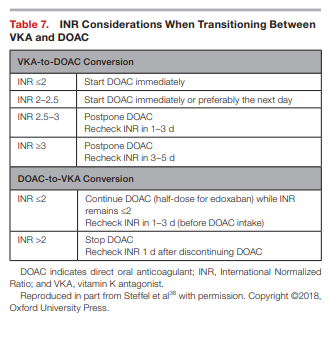
Transitioning between VKA and DOAC and vice
versa requires additional explanation that takes into account the INR measurement. When transitioning from a VKA to a DOAC, the half-life of the VKA compound is imperative to guide therapy. Since warfarin is the principle VKA utilized in the United States, with a half-life of ≈36 to 48 hours, it is advisable to stop warfarin and check INR at least 3 days later. DOACs can be initiated once the INR is ≤2; see Table 7 for management strategies based on INR values.38
When converting from a DOAC to a VKA, providers should administer DOACs concomitantly with VKAs because of the VKAs’ known When converting from a
DOAC to a VKA, providers should administer DOACs concomitantly with VKAs because of the VKAs’ known slow onset of action (≈5–10 days).38 An INR should be rechecked between days 3 and 5 of warfarin therapy. INRs should be checked just before the dose of the DOAC because of their ability to elevate INR values. The INRs should be monitored frequently for at least the first month until INR stability has been achieved (ie, ≥3 consecutive INR readings at goal).Procedural Management
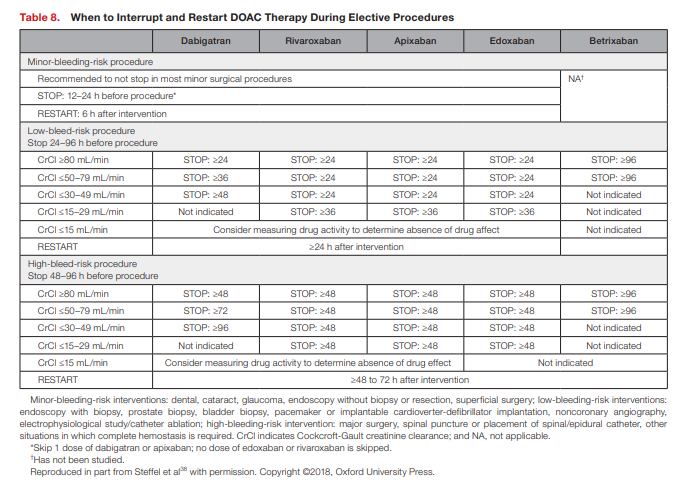
Past guidelines on perioperative and postoperative
management for patients on DOACs have been primarily based on surgical risk (both thrombotic and bleeding), the DOAC’s pharmacokinetic profile, concurrent medications, and patient characteristics (ie, comorbid diseases, history of bleeding complications, renal function, and age).92,93Because of DOACs’ fast onset and offset, periprocedural bridging is generally not required;
however, it is important to note the length of time to
hold regimens before and after procedures.Providers may find it useful to obtain specific coagulation measurements* before procedures, especially in high-risk interventions, to determine the level of hemostasis.
*See Table 5 DOAC Laboratory Monitoring on p. 11 of the PDF.
[For additional information on DOAC monitoring, please see What recommendations are available for laboratory monitoring of direct oral anticoagulants (DOAC)? from the University of Illinois Chicago – Drug Information Group College of Pharmacy Chicago Rockford, Monthly FAQs 2020 July 2020 FAQs.]
However, for the majority of patients undergoing elective procedures, the interruption of DOAC therapy can be substantiated on a time-based approach that takes into consideration bleeding risk of the procedure and renal function (see Table 8).38,93
MAJOR DRUG INTERACTIONS
Drug-drug interactions are an important concern for any patient managed on DOAC therapy.
Concomitantly administered drugs that alter DOAC
plasma concentrations can lead to serious complications, with increased DOAC concentrations potentially resulting in bleeding events, and decrease DOAC concentration placing the patient at risk for thrombus formation. Initially, DOACs were viewed as having minimal drug interactions, which has proven incorrect.When compared with VKAs, which are highly associated with substantial drug-drug interactions, DOACs impart a lower risk; however, they still have significant risk of interactions.
Three different types of drug interactions need to be evaluated when managing DOAC therapy:
(1) agents affecting renal clearance,
(2) agents affecting hepatic clearance, and
(3) agents concurrently affecting hemostasis.
Agents Affecting Renal Clearance
As all DOACs used for treatment of atherothrombosis rely on the kidneys for elimination to varying degrees, agents that hinder this organ’s ability to clear DOAC may place the patient at increased risk for bleeding complications. Common agents to monitor are NSAIDs, diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and immunosuppressants (ie, calcineurin inhibitors such as cyclosporine and tacrolimus, mammalian target of rapamycin inhibitors such as sirolimus and everolimus).38 No DOAC-specific dose adjustments are required but warrant more frequent monitoring of renal function and possibly holding therapy or consideration for transition to an agent with less renal involvement if renal function is compromised.
Agents Affecting Hepatic Clearance
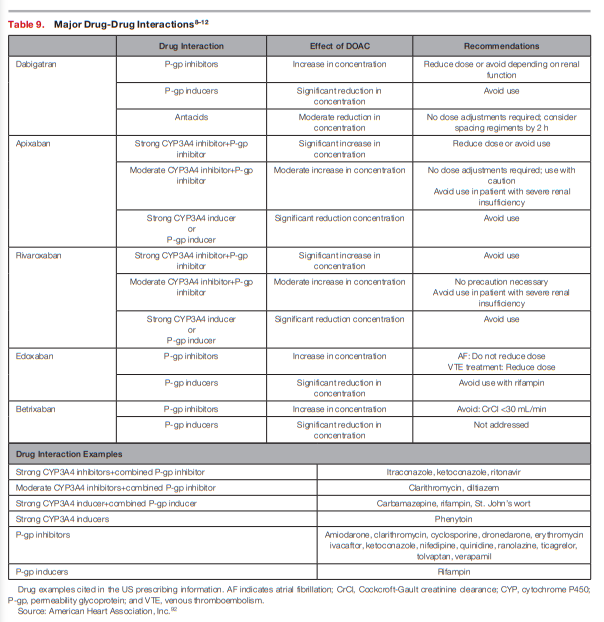
All DOACs are eliminated by either CYP metabolic enzymes* or permeability glycoprotein transporters**; thus, agents that inhibit or induce these enzyme systems can constitute major drug-drug interactions and place the patient at undue risk for complications (see Table 9).
*The Effect of Cytochrome P450 Metabolism on Drug Response, Interactions, and Adverse Effects [PubMed Abstract] [Full Text HTML] [Full Text PDF]. TOM LYNCH, PharmD, AMY PRICE, MD, Eastern Virginia Medical School, Norfolk, Virginia
**P-glycoprotein and its role in drug-drug interactions [Full Text HTML]. Aust Prescr 2014;37:137-9, 4 August 2014.
A common drug-drug interaction example includes
CYP3A4 inhibitors with the use of apixaban, which as
discussed above is the DOAC with the highest reliance
of hepatic biotransformation for clearance. Regimens
such as ketoconazole, ritonavir, and itraconazole significantly reduce apixaban drug concentrations and therefore should be avoided.10 Because of the likelihood of concomitant interacting medications, the risk of drug-drug interactions increases when DOACs are prescribed in patients with the following comorbidities: HIV, organ transplantation, infection, malignancy, epilepsy, and arrhythmia.Refer to previously published reviews for a more comprehensive list of interacting medications and associated management
strategies.38,95Agents Concurrently Affecting Bleeding Risk
Start here.