In this post I link to and excerpt from the chapter, Diagnosis Of Metabolic Acid-Base Disorders, September 12, 2019 of Dr. Farkas’ incredible Internet Book Of Critical Care [Link is to TOC]. And after reviewing the chapter, listen to the 20 minute summary podcast of the chapter.
Dr. Farkas has created this Table of Contents of the chapter and each heading is a direct link to that part of the chapter:
CONTENTS
- Getting started: metabolic vs. respiratory abnormalities
- The anion gap
- Core: Diagnostic approach to metabolic pH abnormalities
- Respiratory pH analysis & how much will it help us?
- Basic principle: Diagnoses are more important than numbers
- Other approaches to pH analysis
- Podcast
- Questions & discussion
- Pitfalls
- PDF of this chapter (or create customized PDF)
All that follows are excerpts from the Diagnosis Of Metabolic Acid-Base Disorders Chapter of The Internet Book Of Critical Care [except where noted – material in brackets]:
getting started: metabolic vs. respiratory abnormalities
metabolic vs. respiratory pH disorders
- Metabolic disorders involve a primary change in the serum bicarbonate and/or anion gap.
- Respiratory disorders involve primary changes in the pCO2 (due to changes in CO2 removal by the lungs).
ABG/VBG isn’t needed to evaluate metabolic pH disorders
- Complete analysis of pH status requires blood gas analysis, but all you need to determine the metabolic pH disorders is an electrolyte panel.
- Analysis of the metabolic pH disorders is usually the most important component (and frequently sufficient to guide treatment).
- Metabolic pH analysis should be performed on every set of electrolytes obtained from every patient. Additional evaluation with ABG/VBG may be performed more selectively.
the anion gap
basic properties of the anion gap
- Anion Gap (AG) = Na – Bicarb – Chloride
- A normal anion gap is roughly 4-12 mM.
- Historically, the normal range of anion gap was often quoted as being higher (e.g. up to ~16 mM). However, with newer electrolyte analyzers, the upper limit of normal has decreased to ~11-12 mM (24766940). This may vary between laboratories however, so the best practice is to be familiar with normal values at your hospital.
- Comparison with a baseline anion gap might be helpful, if that is available. For example, a patient with chronic renal insufficiency may have a chronically elevated anion gap at 14 mM. If this represents no change from previous labs, it is less likely to represent an acute and dangerous process.
you don’t need to correct for albumin
- Using an un-corrected anion gap with a cutoff value of >10 mM has the same performance for detecting anion gap metabolic acidosis as using a corrected anion gap with a higher cutoff value of ~14 mM (16858097, 18431828, 19087326). Different cutoffs may affect sensitivity and specificity, but the overall performance of the test is unchanged (i.e. same area under the Receiver Operator Curve). So correcting for the albumin is an extra step which provides no improved clinical utility to this test.
- Further discussion of this here.
causes of an elevated anion gap
- This generally indicates the presence of an anion-gap metabolic acidosis (AGMA).
- More on the differential diagnosis of this in a forthcoming chapter on AGMA.
performance of anion gap in detecting lactic acidosis
- Anion gap is not reliable for detecting mild degrees of lactic acidosis (e.g. lactate of 2-4 mM). This is because a normal anion gap spans a range of ~10 mM. If the patient begins with a baseline anion gap at the lower limit of normal (e.g. 4 mM), they could easily develop a substantial lactic acidosis while still having an anion gap within the normal range (17699401).
- If there is a specific concern regarding whether the patient might have lactic acidosis, the best strategy is to check lactate directly. An anion gap cannot be relied upon to exclude elevated lactate. However, an anion gap remains a useful surveillance test for the detection of marked lactic acidosis.
causes of a reduced anion gap
- This might be defined as an anion gap below ~4 mM.
- Potential causes
- Laboratory error (possibly the most common). This may occur due to severe hypernatremia, or severe hyperlipidemia (causing pseudohyponatremia).
- Increased levels of cations: hyperkalemia, hypercalcemia, hypermagnesemia, or lithium.
- Elevated levels of immunoglobulins (multiple myeloma, polyclonal gammopathy).
- Cationic drugs (polymixin B), administration of ammonium chloride.
- Pseudohyperchloremia: Bromide or Iodide may cause chloride level to be incorrectly measured as crazy high. A similar phenomenon may occur with salicylates (29344509).
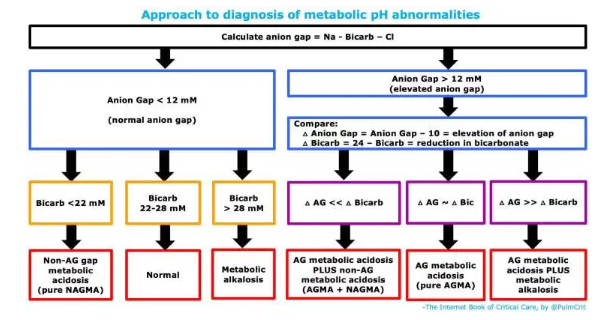
diagnostic approach to metabolic pH abnormalities
1. determination of the anion gap to evaluate for anion gap metabolic acidosis (AGMA)
- More on the anion gap above.
2. if the anion gap is normal, just look at the bicarbonate
- Bicarbonate <22 mM with a normal anion gap indicates a pure non-anion-gap metabolic acidosis (NAGMA).
- Bicarbonate >28 mM with a normal anion gap indicates a pure metabolic alkalosis.
- A bicarbonate of 22-28 mm with a normal anion gap indicates a normal metabolic pH status.
3. if the anion gap is elevated, determine the “delta delta”
- What are these?
- Delta anion gap = (Anion Gap) – 10. This is roughly the degree of elevation of the anion gap.
- Delta bicarbonate = 24 – bicarbonate. This is roughly the degree of reduction of the serum bicarbonate.
- Comparing these values can help determine if there is an additional process, in combination with the anion gap metabolic acidosis. Specifically:
- (1) If delta anion gap is roughly equal to the delta bicarbonate, then no other process is present. This is about what we would expect for an isolated, pure anion gap metabolic acidosis.
- (2) If the delta anion gap is much higher than the delta bicarbonate, then a second process is present which is increasing the bicarbonate level. This reveals a combination of an anion gap metabolic acidosis plus a metabolic alkalosis.
- (3) If the delta anion gap is much lower than the delta bicarbonate, then this reveals a second process which is decreasing the bicarbonate. This indicates a combined anion gap metabolic acidosis plus a non-anion-gap metabolic acidosis.
respiratory pH analysis & how much does this help us?
An ABG/VBG will provide information about respiratory pH abnormalities. Although this is traditionally considered a mandatory component of pH analysis, the amount of useful information provided by this analysis is unclear. Blood gas analysis can answer essentially two questions:
- Is there adequate compensation?
- Is there a primary respiratory disorder?
#1) is there adequate respiratory compensation?
- Overall, it’s murky how information about respiratory compensation should affect our management of critically ill patients. Specifically, decisions regarding intubation or selection of respiratory support devices are generally made on the basis of clinical assessment and diagnosis (not blood gas values).
#2) is there a primary respiratory disorder?
- This is undoubtedly more important than whether there is adequate respiratory compensation (#1).
- If we detect a metabolic pH abnormality, there is a possibility that it represents a secondary compensatory response to a respiratory abnormality (the right side of the chart above). Specifically:
- (a) Metabolic acidosis could be due to a chronic respiratory alkalosis
- (b) Metabolic alkalosis could be due to a chronic respiratory acidosis
- Sorting this out is important, because it leads to an entirely different diagnosis and management.
- From a practical standpoint:
- (a) Metabolic acidosis due to chronic respiratory alkalosis is extremely rare (unless a patient is being mismanaged on mechanical ventilation). This is a bit of a zebra.
- (b) Metabolic alkalosis due to chronic respiratory acidosis is common in patients with hypercapnia of any etiology (most commonly COPD, obesity hypoventilation syndrome, or possibly chronic opioid use). This can generally be diagnosed based on a compatible clinical history, as well as review of archival labs (which show a chronic metabolic alkalosis).
so, what does the blood gas analysis really add to a clinically relevant analysis of pH?
- As explored above, blood gas analysis usually won’t have a substantial effect on our diagnosis and management of the patient (assuming that we have fully evaluated the electrolyte panel and performed a thoughtful history and physical examination).
- Occasionally, blood gas analysis may reveal a chronic respiratory acidosis as the cause of a metabolic alkalosis (2b) – but in most cases this would already have been suspected on the basis of clinical history and/or prior laboratory studies.
- So, in sum, blood gas analysis isn’t mandatory when analyzing a patient’s acid-base status. It may be obtained on a selective basis, rather than broadly being ordered for any patient with a pH abnormality.
etiologies are more important than numbers
types of information from pH analysis
- (1) Diagnostic information – clues as to the underlying diagnosis.
- (2) Severity information – how severe is the pH abnormality?
Generally, the most critical aspect of pH analysis is to identify unexpected diagnoses (#1). The precise severity of the disorder is less important. As long as we have correctly identified the underlying diagnosis and we are treating it appropriately, the exact pH numbers are often relatively unimportant.
Pitfalls
- The anion gap should be calculated whenever evaluating any set of chemistries (if your computer system doesn’t do this for you automatically).
- An anion gap elevation in a critically ill patient should be considered to likely represent lactic acidosis and a life-threatening process, until proven otherwise.
- Many approaches to acid-base physiology make this unnecessarily difficult and confusing. Avoid this. For example, you don’t need to correct the anion gap for albumin, potassium, or anything else.