In this post I link to and excerpt from Dr. Josh Farkas’ Internet Book of Critical Care chapter Septic Shock, February 7, 2017 by Josh Farkas.
Note to myself: I create these excerpts because doing so helps me to fix the material in my mind. But for review, I will be using the show notes from Dr. Farkas’ Septic Shock chapter.
Here are excerpts:
CONTENTS [The links below go directly to that section of Dr. Farkas post]
- Front matter
- Definition of septic shock
- Diagnosis
- Source of septic shock & evaluation for source
- Treatment
- Interventions of no real value
- Resuscitative endpoints ??
- Algorithms
- Podcast
- Questions & discussion
- Pitfalls
- PDF of this chapter (or create customized PDF)
preamble
This chapter describes my approach to septic shock. It is evidence-based, but there’s inadequate evidence available to prove that it is superior to innumerable other ways of treating septic shock. Overall, it is a fairly simple approach that doesn’t involve a lot of fancy gadgetry. As such, this approach should be accessible to a wide variety of practitioners.
brief history of septic shock treatment
Please review this historical material in the link above.
definition of septic shock
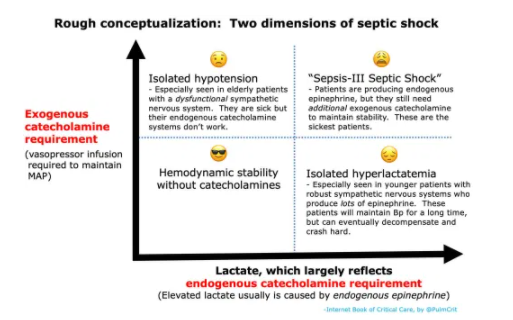
The consensus definition of septic shock was updated from Sepsis-II to Sepsis-III recently. These definitions may be summarized as follows:
- Sepsis II definition of septic shock: Infection causing persistent hypotension, despite fluid resuscitation.
- Sepsis III definition of septic shock: Infection causing vasopressor requirement to maintain a MAP > 65 mm (despite fluid resuscitation) plus a serum lactate >2 mM.
Neither of these definitions is perfect, but they focus our attention on two key bits that together help delineate septic shock:
- Bit #1: Overt hypotension
- Bit #2: Hyperlactatemia – this is generally a reflection of aerobic lactate production due to endogenous epinephrine. It may be grossly conceptualized here as a measurement of the patient’s endogenous epinephrine release.
Sepsis III requires both bits, which is a mistake because they measure different things:
Lactate is not specific to septic shock. Lactate may be elevated by a panoply of conditions (including any shock state, physiologic stress, beta-agonists, seizure, or hepatic dysfunction). Elevated lactate does often identify patients who are at increased risk of mortality, who require more intensive investigation and treatment.
Ultimately, formal definitions are more relevant to clinical trials than bedside management of individual patients. These blunt tools are inadequate to direct patient management. Instead, a diagnosis of septic shock should be made carefully on an individual basis, using clinical judgement and consideration of the following factors:
- (1) Type of underlying infection
- Some infections (e.g. necrotizing fasciitis, ascending cholangitis) are more likely to cause septic shock. There should be a lower threshold to diagnose septic shock and initiate aggressive management in these patients.
- (2) Degree of hemodynamic instability
- Shock index (heart rate / systolic blood pressure)
- Blood pressure, compared to patient’s baseline pressure
- Vasopressor requirement
- Evidence of end-organ hypoperfusion (e.g. urine output, skin perfusion)
- (3) Degree of hyperlactatemia
- Presence of other factors which may increase lactate levels (e.g. albuterol, hepatic dysfunction).
- Lactate >4 mM suggests a significant mortality (28248722).
- (4) Other end-organ failures (e.g. delirium, shock liver)
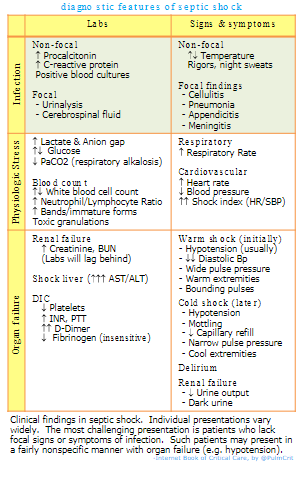
clinical presentations
Septic shock encompasses a broad range of infections in a diverse range of patients. The table below shows common signs and symptoms of sepsis. This can be a difficult diagnosis, because different patients will present with different constellations of these findings.
In many cases, septic shock may be suspected before the underlying infection is definitively diagnosed. In such cases, it is generally best to empirically treat as if the patient has septic shock, while obtaining additional information (e.g., culture data, definitive CT imaging).
- For patients who are clinically in shock with no obvious cause (despite evaluation of history, physical exam, and echocardiography), there should be a high index of suspicion for septic shock.
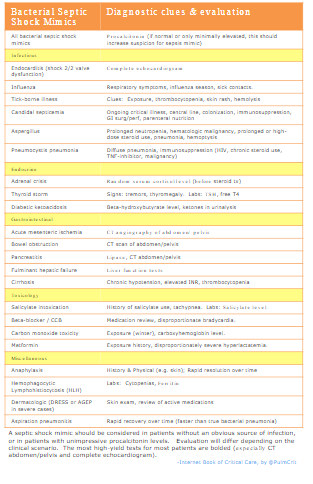
sepsis mimics
With increased focus on sepsis and septic shock, other disorders are increasingly likely to be misdiagnosed as septic shock (27692840). Common mimics and their evaluation are shown here:
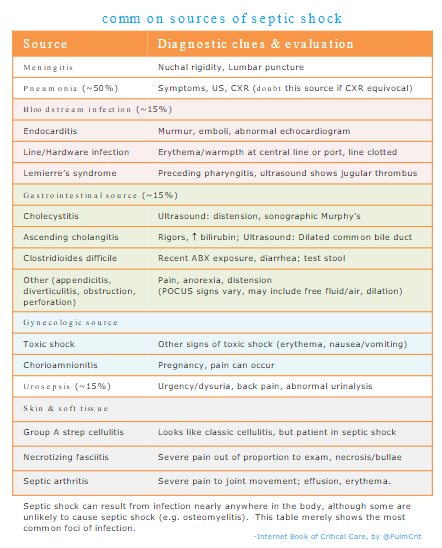
common sources & evaluation of source
source evaluation
- History
- ? Localizing symptoms
- ? Travel to areas with endemic infections (e.g. tick-borne infections, malaria)
- ? Hardware (e.g. prosthetic joints, pacemakers, implanted ports, central lines)
- Physical exam: see figure above
- Labs
- Basic labs: Chem-10 (including Ca/Mg/Phos), CBC with differential, Coags, Liver function tests
- Cultures: Blood cultures x2, culture of any indwelling line present >48 hours
- Procalcitonin
- Urinalysis and culture
- Specific tests directed at other potential infections (e.g. PCR for anaplasma and ehrlichia; more here)
- Imaging
- Chest X-ray
- Low threshold to obtain CT abdomen/pelvis if source of infection remains unclear. Especially in elderly patients, severe abdominal pathology may occur with minimal findings on history and physical exam. Consider a CT angiogram if mesenteric ischemia is possible.
- Additional tests as clinically warranted, for example:
- CT head and lumbar puncture if altered mental status or nuchal rigidity
- Ultrasound evaluation of any potential sites of infection
- Paracentesis if ascites (even if previously present)
vasopressors
increase MAP>65mm promptly with peripheral vasopressor infusion
- Correlational evidence suggests that a longer duration of hypotension increases the risk of renal failure.
- The traditional strategy of flogging the patient with fluid for hours before starting pressors is ill-conceived.
- Peripheral vasopressor may be started without delay to support the blood pressure.
- If the patient improves following fluid resuscitation, then pressor may be weaned off.
- If vasopressor requirements escalate, then transition to a central line may be considered
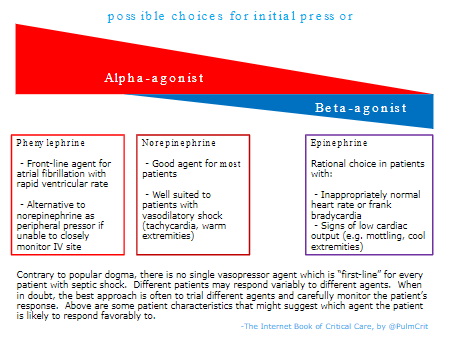
choice of the first pressor: there is no “first-line” agent
- Traditional dogma favors a specific sequence of vasopressors for all patients (typically beginning with norepinephrine, then with sequential addition of vasopressin, and finally epinephrine). Evidence doesn’t really support this:
- Initial vasopressor support with vasopressin was potentially beneficial in the VANISH trial (27483065). This shows that a pure vasoconstrictor can be used as a front-line pressor for septic shock.
- Epinephrine was shown to yield similar results to norepinephrine in the CAT trial 😺 (18654759) .
- The only prospective study comparing norepinephrine to phenylephrine found nearly identical hemodynamic responses (19017409).
- For most patients, norepinephrine is a good choice. However, the selection of pressors may be individualized based on patient physiology, as shown below.
- The only pressor which shouldn’t be used is dopamine (based on evidence of harm in prospective RCTs)(20200382, 26323041).
peripheral vasopressors
- The main concern with peripheral vasopressor infusion is extravasation into the skin leading to necrosis. Fortunately, this is rare. Overall, the risk of skin necrosis should never be prioritized over the risk of systemic hypoperfusion and death.
- Skin necrosis is both less common and less important than mortality and systemic hypoperfusion.
- Norepinephrine is safe for peripheral infusion, but the risk of extravasation does increase if infusions are used for prolonged periods of time (25669592). Peripheral norepinephrine has been validated in a medical ICU with a rigorous protocol for placing IV lines and monitoring their function closely (26014852).
- Phenylephrine or epinephrine are the safest agents to use peripherally. Both agents have been administered via a subcutaneous route intentionally in the past. There appear to be no reports in the literature of these agents’ causing skin necrosis. It’s sensible to avoid infusion into the wrist or hand, but overall these agents are unlikely to cause necrosis if extravasation occurs.
- Note that phenylephrine infusion is physiologically very similar to norepinephrine.
- Vasopressin administration peripherally should probably be avoided. If vasopressin extravasates, there is no way to counteract its effect (unlike catecholamine extravasation, which can be treated with local infiltration with phentolamine).
vasopressin
- Vasopressin is a non-catecholamine pure vasoconstrictor that may improve renal function (compared to norepinephrine).
- Drawbacks of vasopressin:
- Hard to titrate (half-life of ~30 minutes, so it will take a long time to reach steady state). Vasopressin can be used as a titratable pressor at doses ranging from 0-0.06 U/min, but don’t expect dose adjustments to have an immediate effect (27483065).
- The combination of vasopressin plus norepinephrine can cause digital ischemia and necrosis.
- How to use vasopressin?
- Best for patients with warm extremities (vasodilatory shock) and acute kidney injury.
- Monitor extremity perfusion and stop vasopressin immediately if digital ischemia occurs.
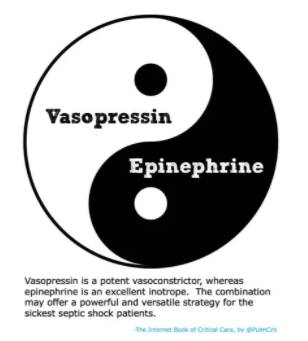
- Consider combining vasopressin with epinephrine (rather than norepinephrine). Theoretically, this may allow taking advantage of the renal-perfusion benefits of vasopressin while avoiding excessive vasoconstriction.
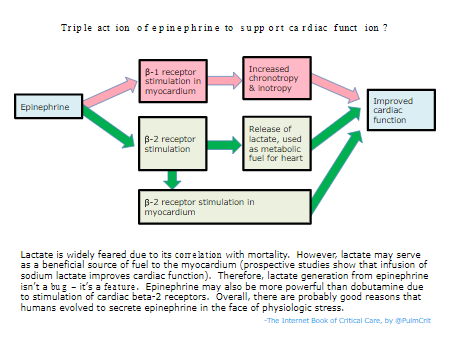
epinephrine might be the preferred inotrope for septic shock
- Traditionally, there has been a preference for catecholamine inotrophes that selectively affect the beta-1 receptor. This is based on the following two concepts, which are both wrong:
- Epinephrine may actually have advantages over dobutamine:
- (1) Epinephrine may have greater efficacy as an inotrope (figure below).
- (2) Dobutamine causes vasodilation, which may decrease the blood pressure and exacerbate vasodilatory shock. This can cause problems if the dobutamine isn’t titrated very thoughtfully. In contrast, epinephrine contains alpha-adrenergic stimulation which prevents it from having a net vasodilatory effect.
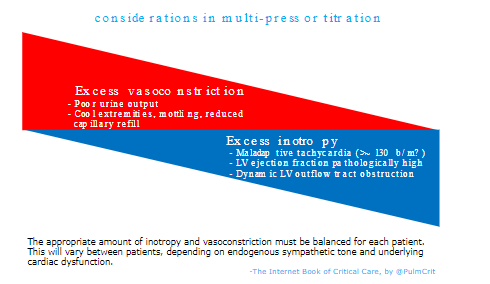
multi-pressor titration
- Titration of multiple vasopressors involves avoiding excessive vasoconstriction or excessive inotropy (figure below). The optimal balance will vary between patients.
- When in doubt, the best approach is often to empirically up- and down-titrate pressors in order to sort out what the various agents are doing.
- If a patient strongly responds to up/down titration of an agent, that drug is more likely to be causing benefit.
- If a patient has no/minimal response to adjusting the dose of an agent, that drug probably isn’t helping much (and may be causing harm).
- Norepinephrine down-titration: In addition to adding pressors, it’s also useful to down-titrate pressors which aren’t helping. Sometimes norepinephrine may over-constrict patients, leading to excessive afterload and a drop in ejection fraction (the heart is unable to tolerate the excessive afterload). If adjusting the norepinephrine dose doesn’t affect blood pressure much, that suggests that the norepinephrine dose is excessive. The goal should always be to use the minimal dose of vasopressor(s) necessary to achieve hemodynamic targets.
epinephrine challenge
- Contraindications to epinephrine challenge:
- Significant tachycardia (e.g. heart rate >120 b/m)
- Echocardiography shows the left ventricle is already hyperkinetic.
- Indications for epinephrine challenge
- (1) Hypoperfusion (e.g. poor urine output, cold extremities, mottling, poor capillary refill)
- -plus, ideally-
- (2) Some indicator that epinephrine might help, for example
- i) Bedside echo shows ejection fraction is reduced or (inappropriately) normal
- ii) Heart rate is inappropriately slow or normal (e.g. <80 b/m)
- iii) Lactate is inappropriately low relative to the severity of illness (inappropriate normolactatemia, suggesting an inadequate endogenous sympathetic response)
- How to perform: Start an epinephrine infusion at ~4-5 mcg/min.
- Judging response to the epinephrine challenge:
- (1) Effect on skin perfusion (e.g. capillary refill time, mottling, and temperature).
- (2) Effect on blood pressure. On average, epinephrine has a relatively equivalent effect on blood pressure compared to norepinephrine (e.g. 1 mcg/min epinephrine ~ 1 mcg/min norepinephrine). If the epinephrine has a considerably greater effect on blood pressure than norepinephrine, this suggests that it’s helping (e.g. adding 4 mcg/min epinephrine allows the norepinephrine dose to be decreased by 10 mcg/min).
- (3) Effect on heart rate – an excessive tachycardic response to epinephrine may be harmful.
- Follow-up:
- If the patient responds well to epinephrine, then this should be continued. In many cases, other vasopressors may be down-titrated (try to achieve MAP and perfusion targets with the minimal cumulative dose of vasopressor).
- If the patient responds poorly to epinephrine, then stop it.
going further…
- Early norepinephrine to stabilize the MAP
- Epinephrine challenge
- Peripheral pressors
antibiotics
Start here.