Today, I review, link to and excerpt from Clinical Diabetes‘ Recommendations for Practical Use of Metformin, a Central Pharmacological Therapy in Type 2 Diabetes [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Inês H. Vieira; Luísa M. Barros; Carla F. Baptista; Dírcea M. Rodrigues; Isabel M. Paiva. Clinical Diabetes January 2022, Vol.40, 97-107. doi:https://doi.org/10.2337/cd21-0043.
All that follows is from the above review.
When administered orally, metformin is absorbed mainly in the small intestine, with a bioavailability of 55 ± 16%. The drug is eliminated unchanged by the kidney, with an average half-life of 5 hours in individuals with normal renal function (8).
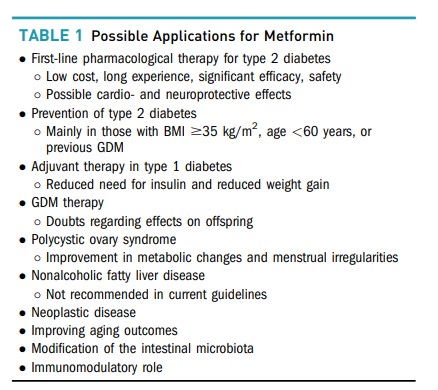
Metformin currently plays a central role in the treatment of type 2 diabetes and may also have benefits in other pathologies. Although it is generally considered to have a good safety profile, some precautions are essential for its correct use. This article reviews the advantages and applications of metformin, but its main focus is on the drug’s adverse effects and on providing practical recommendations for its use.
Role of Metformin
Type 2 Diabetes
Metformin has considerable efficacy in reducing A1C (by ∼1.12% as monotherapy and 0.95% when added to other drugs) (11). The UK Prospective Diabetes Study (UKPDS) documented its beneficial effects on glycemic control, which were similar to those obtained in groups treated with a sulfonylurea or insulin (12,13). However, metformin monotherapy in patients with type 2 diabetes and overweight/obesity yielded a greater reduction in all-cause mortality and other diabetes-related end points with less hypoglycemia and without inducing weight gain (13).
Several trials have also shown glycemic benefits from combination therapy with metformin and pioglitazone (16–18), DPP-4 inhibitors (19–22), GLP-1 receptor agonists (21,23,24) and sodium–glucose cotransporter 2 inhibitors (25–29).
A meta-analysis comparing metformin with this wider range of noninsulin antidiabetic agents found similar efficacy on glycemic control for all classes in monotherapy and in combination with metformin, except for DPP-4 inhibitors, which was less effective) (30).
The dosing of metformin for maximum benefit has been evaluated in a few studies. With doses ranging from 500 to 3,000 mg, there seems to be a dose-dependent effect on glycemic control, mainly on FPG (46,47).
Potential Adverse Effects
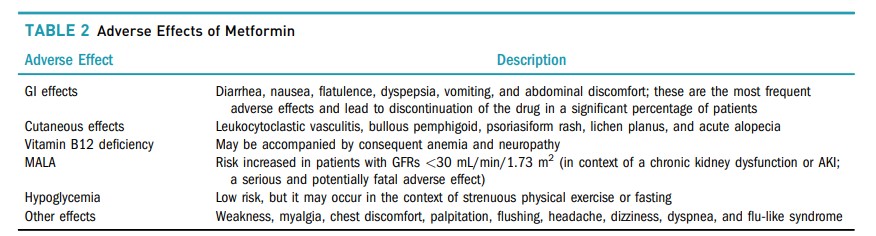
Adverse effects of metformin are common, but mostly not serious, with GI intolerance being the most frequently described, occurring in >20–30% (71). Diarrhea is especially common, with reported incidences varying from 20 to 60%. Nausea, flatulence, dyspepsia, vomiting, and abdominal discomfort are also frequently reported. These side effects may lead to nonadherence in a significant proportion of affected patients (72).
Vitamin B12 deficiency is also a possible side effect of metformin, likely because of malabsorption.
In an analysis of the DPP/DPPOS (77), there was a 13% increase in the risk of B12 deficiency for each year of metformin use. Increased levels of homocysteine, suggesting deficiency at the tissue level, and a higher prevalence of anemia and neuropathy were also detected.
Metformin-associated lactic acidosis (MALA) is the most feared complication. Experience with other biguanides such as phenformin and buformin contributed to this concern, as LA was much more frequent with these drugs, which were ultimately withdrawn from the market (80,81), than with metformin. However, this concern persists, and it is important to recognize who may be truly more susceptible to this complication.
In 2016, because of the accumulation of data such as those mentioned above, first the U.S. Food and Drug Administration (FDA) (91) and later the European Medicines Agency (EMA) (92) extended the use of metformin in individuals with a GFR >30 mL/min/1.73 m2. For those with a GFR <30 mL/min/1.73 m2, an increased risk of MALA continues to be described (93,94).
Because the liver is responsible for most lactate elimination, there is also the possibility that the use of metformin is not safe in the setting of chronic liver disease (95), and the drug is often discontinued in cirrhotic individuals (96). Conversely, in a retrospective evaluation of 250 individuals diagnosed with cirrhosis, continuation of metformin was associated with a 57% reduction in the risk of death, which was limited to those with nonalcoholic steatohepatitis–related cirrhosis (96). In a comparison of different therapies for diabetes in 100 individuals with cirrhosis caused by hepatitis C virus, metformin therapy was associated with a lower occurrence of hepatocellular carcinoma and death associated with liver disease (97). More recently, Smith et al. (95) found that both metformin and lactate levels remained in the safe range (<5 mg/L and 5 mmol/L, respectively) in a group of individuals with chronic liver disease, suggesting the safety of metformin therapy in this group of patients (95). These studies, however, make no specific mention of the use of metformin in patients who maintain excessive alcohol consumption.
Crowley et al. (98) performed a meta-analysis on the use of metformin in settings in which caution was traditionally recommended. When compared with the alternatives, metformin’s use in the setting of chronic kidney disease (CKD) was associated with 22% lower mortality and less hypoglycemia. In patients with heart failure, 20% less mortality and a 13% reduction in heart failure–related readmissions were found. Available data on chronic liver failure did not allow the performance of meta-analysis; however, a tendency toward lower mortality was observed in the included studies (98).
Hypoglycemia is one of the adverse effects most feared by people with diabetes. By not directly stimulating insulin secretion, metformin has a low risk of hypoglycemia. Nevertheless, it can occur in the context of intense physical activity or fasting (99).
Other adverse reactions include weakness, myalgia, chest discomfort, palpitation, flushing, headache, dizziness, dyspnea, and flu-like syndrome. Skin side effects have also been reported, including leukocytoclastic vasculitis, bullous pemphigoid, psoriasiform rash, lichen planus, and acute alopecia (100). The adverse effects of metformin are summarized in Table 2.