In addition to today’s resource, please see and review:
- Links To And Excerpts From 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain
Posted on September 12, 2023 by Tom Wade MD - the outstanding YouTube lecture, The Role of Coronary CTA in the New Guideline Era, from Yale Cardiovascular Medicine Grand Rounds, 1:07:31, Jan 11, 2022, by Todd C. Villenes, MD.
Today, I reviewed, link to, and excerpt from the 2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department: A Report of the American College of Cardiology Solution Set Oversight Committee [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. J Am Coll Cardiol. 2022 Nov 15;80(20):1925-1960. doi: 10.1016/j.jacc.2022.08.750. Epub 2022 Oct 11.
The above resource has been cited by:
-
Occlusive myocardial infarction – emerging paradigm of acute coronary syndromes.
Postepy Kardiol Interwencyjnej. 2023 Jun;19(2):186-187. doi: 10.5114/aic.2023.127903. Epub 2023 Jun 5.PMID: 37465623 Free PMC article. No abstract available. -
Troponin in early presenters to rule out myocardial infarction.
Eur Heart J. 2023 Aug 7;44(30):2846-2858. doi: 10.1093/eurheartj/ehad376.PMID: 37350492 Free PMC article. -
Am Heart J Plus. 2023 Mar;27:100265. doi: 10.1016/j.ahjo.2023.100265. Epub 2023 Feb 3.PMID: 36779177 Free PMC article.
-
Cureus. 2023 Jan 1;15(1):e33227. doi: 10.7759/cureus.33227. eCollection 2023 Jan.PMID: 36601361 Free PMC article.
-
Wellens Syndrome: A Possible Precursor.
Cureus. 2022 Nov 28;14(11):e31963. doi: 10.7759/cureus.31963. eCollection 2022 Nov.PMID: 36582578 Free PMC article.
All that follows is from the above resource.
Outline
- Key Words
- Solution Set Oversight Committee
- Table of Contents
- Preface
- 1. Introduction
- 2. Methods
- 3. Definitions and Assumptions
- 4. Summary Graphic
- 5. Description and Rationale
- 6. Conclusions and Implications
- President and Staff
- Appendix
- Appendix 1. Author Relationships With Industry and Other Entities (Relevant)—2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department
- Appendix 2. Peer Reviewer Relationships With Industry and Other Entities (Comprehensive)—2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department
- Appendix 3. Abbreviations
- References
All that follows is from the 2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department: A Report of the American College of Cardiology Solution Set Oversight Committee [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. J Am Coll Cardiol. 2022 Nov 15;80(20):1925-1960. doi: 10.1016/j.jacc.2022.08.750. Epub 2022 Oct 11.
1. Introduction
Chest pain is one of the most common reasons for emergency department (ED) visits, accounting for over 7 million ED visits annually.2 It is one of the most challenging conditions to evaluate, which contributes to ED overcrowding, inefficient use of resources, and delays to diagnosis. A major challenge is to rapidly identify the small number of patients who have acute coronary syndrome (ACS) or other life-threatening conditions among the large number who have more benign conditions, many of which are noncardiac.3,4
Over the last 40 years, considerable efforts have been made to streamline and improve the chest pain evaluation process. Successive iterations of evaluation and management strategies have reduced both the number of patients who require admission as well as ED length of stay. The objectives of this Expert Consensus Decision Pathway are to provide structure around the evaluation of chest pain in the ED and to facilitate rapid disposition and limit unnecessary testing among patients with chest pain who are at low risk and who do not have ACS. The document also aims to provide critical appraisal of the options for clinical decision pathways (CDPs) that hospitals may choose from to achieve these aims. Implementation of accelerated CDPs has the potential to further reduce ED length of stay and increase the proportion of patients who are eligible for rapid ED discharge and do not routinely require additional diagnostic testing, without compromising patient safety.
3. Definitions and Assumptions
To limit inconsistencies in interpretation and to develop guidance that is complementary to current evidence-based guidelines for the management of chest pain in the ED, specific definitions and assumptions were considered by the writing committee in the development of the consensus recommendations.
3.1. Definitions
- 1. hs-cTn assays: Assays for cardiac troponin (cTn) T or I that meet the following criteria: assay imprecision (ie, coefficient of variation [CV]) at the 99th percentile value ≤10%; and at least 50% of apparently healthy men and women have cTn concentrations above the assay’s limit of detection (LoD).7 However, not all assays designated as hs-cTn by the United States Food and Drug Administration (FDA) meet these measurement criteria, particularly in women.8
- 2. CDPs: These are structured protocols for evaluation of patients with suspected ACS using hs-cTn assays. They include serial measurements of hs-cTn at specific timepoints and are designed to allow safe disposition of low-risk patients with chest pain in an expedited and efficient manner.
- 3. Efficacy: In studies evaluating performance of CDPs, efficacy is defined as the proportion of individuals meeting “rule-out” criteria based on the CDP algorithm.
- 4. Limit of blank (LoB): This is the highest apparent cTn concentration found with a given assay when testing replicates of a sample known to contain no cTn (ie, blank sample).
- 5. LoD: This is the lowest cTn concentration that can be reliably distinguished from the LoB when testing replicates of samples known to contain cTn.
- 6. Limit of quantification (LoQ): This is the lowest cTn concentration that can be reported reliably as a number, based on a CV ≤20%, as per FDA regulations.
- 7. Minimally elevated hs-cTn or minor elevations in hs-cTn: hs-cTn values above the LoQ but below the 99th percentile upper reference limit (URL).
- 8.vElevated hs-cTn: hs-cTn values above the 99th percentile.
- 9. Relative change (Δ) in hs-cTn: the percentage change in hs-cTn across serial measurements. Relative changes ≥20% are considered significant and indicative of acute myocardial injury. However, at low troponin concentrations near the 99th percentile URL, absolute (rather than relative) change values provide greater specificity for acute myocardial injury.
- 10. Absolute change (Δ) in hs-cTn: The change in hs-cTn across serial measurements, reported as an absolute value in ng/L. At low hs-cTn concentrations near the 99th percentile URL, absolute rather than relative Δ should be used. Values are assay dependent. Recommended CDPs use absolute rather than relative Δ values.
- 11. Nonischemic electrocardiogram (ECG): ECGs that are normal, have nonspecific findings, left ventricular hypertrophy with or without repolarization abnormalities, left or right bundle branch block, or paced rhythm (not meeting Sgarbossa9 or Modified Sgarbossa10,11 criteria for myocardial infarction [MI]).
3.2. General Clinical Assumptions
- 1. The content of this Expert Consensus Decision Pathway applies only to patients presenting to the ED with chest pain or other symptoms suggestive of myocardial ischemia undergoing evaluation for possible ACS. The Expert Consensus Decision Pathway does not apply to patients with stable angina or those evaluated in settings other than the ED. For these other patient groups, the 2021 AHA/ACC/multisociety chest pain guideline provides comprehensive recommendations on chest pain evaluation and management not limited to the ED setting.5 This Expert Consensus Decision Pathway is not applicable to patients with hemodynamic instability, significant heart failure, or other conditions that would mandate hospital admission.
- 2. The document is focused on the rapid evaluation and disposition of patients with possible ACS in the ED. It does not address the evaluation and management of patients with definite ACS or to serve as a guide for the diagnosis or management of MI. Readers are referred to the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction (STEMI),12 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes (NSTE-ACS),13 and the Fourth Universal Definition of MI14 for comprehensive recommendations on these topics.
- 3. This Expert Consensus Decision Pathway is focused on CDPs using high-sensitivity cardiac troponin I (hs-cTnI) assays. The pathways are not appropriate for use with older-generation, less-sensitive assays. An important secondary objective of this document is to support the transition to hs-cTn assays, which offer important advantages for the rapid evaluation and disposition of chest pain in the ED and are recommended by the 2021 AHA/ACC/multisociety chest pain guideline.5 It is recommended that U.S. centers transition to hs-cTn assays for optimal patient care.
4. Recommendations regarding noninvasive testing should be considered in the context of availability of institutional testing and local expertise. However, coronary computed tomography angiography (CTA) is an important tool for evaluation of intermediate-risk patients in the ED, and thus, broader application across centers and greater availability within centers is recommended.
- 5. CDPs for rapid evaluation of chest pain should be interpreted within the context of all available clinical information. The provider’s clinical judgment at the bedside remains an indispensable tool that may lead to different triage decisions than those suggested by the CDP.
- 6. The rule-out cutpoints for high-sensitivity cardiac troponin T (hs-cTnT) and hs-cTnI assays recommended in this document may differ slightly from those reported in studies evaluating accelerated CDPs, because we have synthesized data across multiple studies and used concentration cutoffs permitted for reporting in the United States by the FDA. The CDPs included in this document use the LoQ (see definition in the previous text) permitted by the FDA for the 0-hour rule-out criterion.
- 7. The recommendations in this document are based on available data, much of which is observational rather than from randomized controlled trials. As new, relevant, and sound data become available, modifications to CDPs may be necessary.
- 8. Successful implementation of the CDPs outlined in this document requires engagement of a multidisciplinary team with collaboration among emergency medicine, laboratory medicine, cardiology, and hospital medicine specialties. Clinicians caring for patients in whom hs-cTn assays are used need to be aware of the clinical decision thresholds as well as the strengths and limitations of the CDP. Specific recommendations for transitioning to hs-cTn assays have been outlined previously.15
4. Summary Graphic
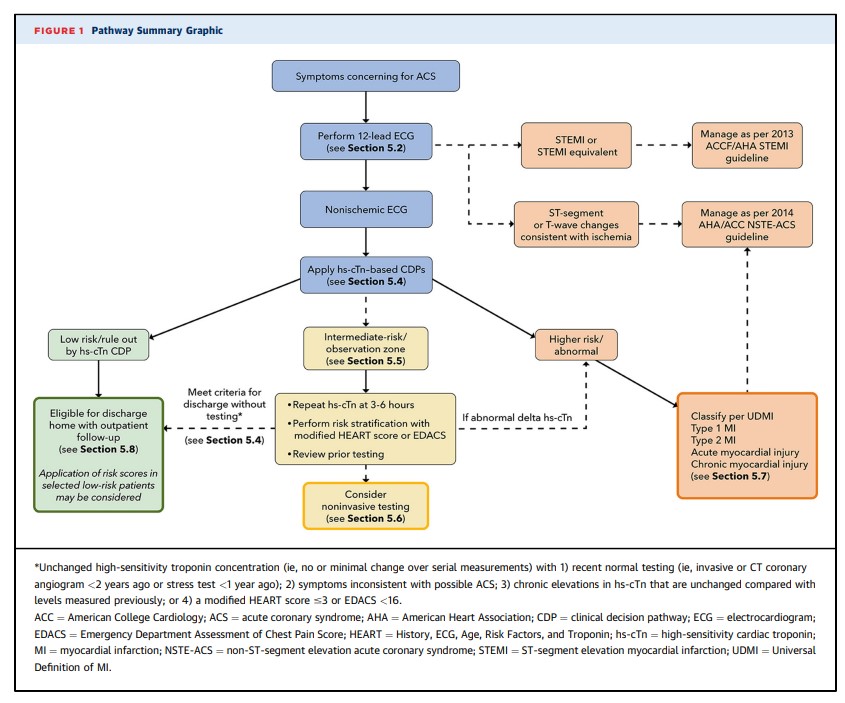
This Expert Consensus Decision Pathway is designed to parallel the usual course of evaluation of patients in the ED with symptoms requiring evaluation for possible ACS (see Figure 1). The first step is careful evaluation of the ECG (see Section 5.2). Patients with a nonischemic ECG can enter an accelerated CDP designed to provide rapid risk assessment and exclusion of ACS (see Section 5.4). Patients classified as low risk (rule out) using hs-cTn–based CDPs supported by this document can generally be discharged directly from the ED without additional testing, although outpatient testing may be considered in selected cases. In contrast, patients with substantially elevated initial hs-cTn values or those who have significant dynamic changes over 1 to 3 hours are assigned to the abnormal/high-risk category and should be further classified according to the Universal Definition of MI into type 1 or 2 MI or acute or chronic nonischemic cardiac injury (see Section 5.7). High-risk patients should usually be admitted to an inpatient setting for further evaluation and treatment. Patients determined to be intermediate risk with the CDP should undergo additional observation with repeat hs-cTn measurements at 3 to 6 hours and risk assessment using either the modified History, ECG, Age, Risk Factors, and Troponin (HEART) score or the ED Assessment of Chest Pain Score (EDACS) (see Section 5.5). Noninvasive testing should be considered for the intermediate-risk group unless low-risk features are identified using risk scores or noninvasive testing has been performed recently with normal or low-risk findings. Details of the assessment steps are provided in Section 5.
5. Description and Rationale
5.1. Initial Evaluation
The initial clinical evaluation of a patient with acute chest pain should focus on rapid identification and treatment of patients with life-threatening conditions such as ACS, aortic dissection, and pulmonary embolism. Patients who are hemodynamically unstable, have significant arrhythmias, or have evidence of significant heart failure should be evaluated and treated appropriately and are not candidates for an accelerated CDP.
There are no physical examination findings specific for coronary ischemia; thus, the examination should be targeted to identify findings associated with high risk for morbidity and mortality in ACS, or to the presence of potential alternative diagnoses. High-risk examination findings include signs of low cardiac output (tachycardia, hypotension, cool extremities, low urine output, and altered mental status), heart failure (pulmonary edema, elevated jugular venous pressure, and peripheral edema), and a new systolic murmur concerning for acute mitral regurgitation or a ventricular septal defect. Clues to non-ACS causes for the patient’s symptoms include fever (endocarditis, pneumonia), pulse differential (aortic dissection), abnormal lung findings (pneumonia, pneumothorax), and abnormal cardiac findings such as a pericardial friction rub (pericarditis) or other murmurs (aortic stenosis, outflow tract obstruction, endocarditis).
As part of the initial assessment, a chest X-ray should
be performed in almost all patients with possible ACS,
given the potential to identify high-risk findings such as pulmonary edema, as well as to identify potential noncardiac causes for the patient’s symptoms. Performance of a chest X-ray should not delay emergent interventions such as primary percutaneous coronary intervention for those with a definitive STEMI.5.2. Initial Evaluation: Focus on ECG
5.2.1. Initial ECG Interpretation
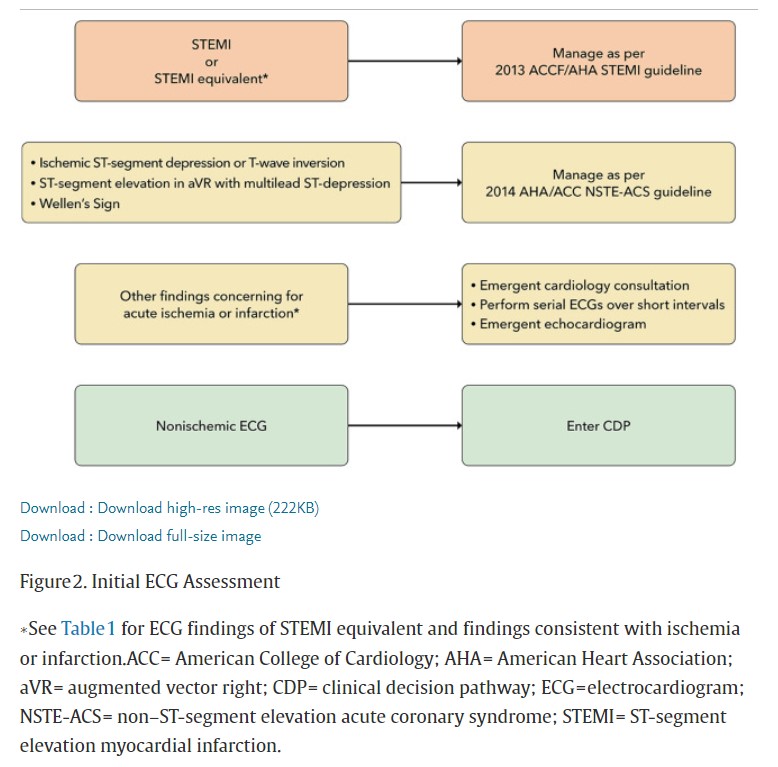
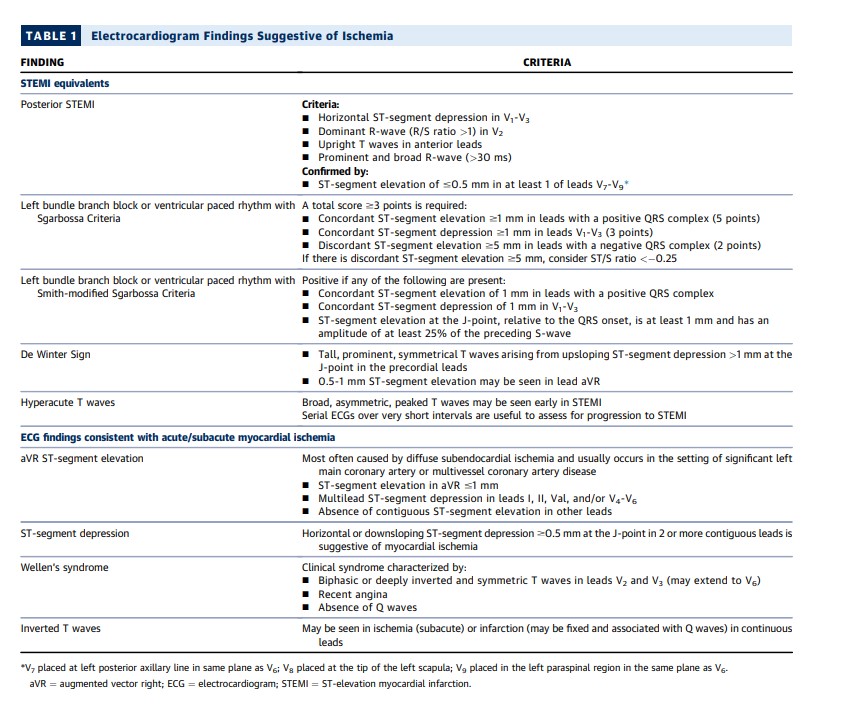
The ECG is critical for the initial assessment and management of patients with potential ACS and therefore should be performed and interpreted within 10 minutes of arrival at the ED.5,12,13 In patients who arrive via emergency medical service transport, the pre-hospital ECG should be reviewed, because ischemic changes may have resolved before ED arrival. In the ED, the initial ECG should be examined for signs of ischemia (see Figure 2), particularly for STEMI or a STEMI equivalent (see Table 1), as this identifies patients who should undergo immediate reperfusion therapy and be managed in accordance with the 2013 STEMI guideline.12 Automated ECG algorithms provide an immediate interpretation and diagnostic assistance, particularly for the inexperienced ECG reader, and may identify subtle ECG changes that just meet STEMI criteria, particularly with inferior ST-segment elevation. However, interpretation accuracy varies among different algorithms, with up to a 2-fold variation in identification of ECGs concerning for ACS.16 Unfortunately, physician accuracy for determining ischemic ECG changes is also variable, with lower sensitivity for smaller degrees of ST-segment elevation.17
Link to 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes
A Report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesIn the absence of ischemic ST-segment elevation, the
ECG should be examined for other changes that have been
associated with coronary artery occlusion (see Table 1)
18-21; when present, these should prompt evaluation for emergent coronary angiography.For patients suspected of ACS who have ST-segment or
T-wave changes suggestive of ischemia, comparison with
previous ECGs can be helpful.22 Emergent consultation for
expert over-read should be obtained for ECGs concerning
for ACS that lack clear diagnostic criteria.A posterior ECG should be performed if the initial ECG is
nondiagnostic but suspicion for a posterior MI is high (see
Section 5.2.2 and Table 1).Emergent transthoracic echocardiography (TTE) for assessment of wall motion should be considered in patients with ECGs concerning for but not diagnostic of ischemia and infarction, particularly when borderline ST-segment elevation or left bundle branch block (LBBB) or equivocal signs of posterior MI are present. Because accurate assessment of wall motion is
difficult, the TTE should be performed and reviewed by a
clinician qualified in echocardiography (see Section 5.6).Finally, the ECG should be reviewed for other findings
suggesting alternative causes for the patient’s symptoms,
such as pericarditis or pulmonary embolism. The absence
of ischemic ECG changes identifies patients at relatively
lower (although not necessarily low) risk for MI and
ischemic complications but is not sufficient for excluding
ACS25,26; therefore, these patients are appropriate for
evaluating using a CDP.All CDPs (see Section 5.4) exclude patients with ischemic ST-segment elevation; however, many do not specifically exclude those with other ECG findings potentially associated with ischemia.27 We recommend that patients with new ischemic ECG changes should be considered high risk and undergo evaluation and treatment according to current NSTE-ACS guidelines*13 and not be entered into an accelerated CDP. [Emphasis added]
*2020 ESC NSTE-ACS Guidelines: Evolving Approaches and Recommendations
May 20, 2021 | Michael C. Kontos, MD, FACC; George W. Vetrovec, MD, MACCExpert Analysis*2020 ESC NSTE-ACS Guidelines: Key Points
Aug 29, 2020 Debabrata Mukherjee, MD, FACC*2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal, Volume 42, Issue 14, 7 April 2021, Pages 1289–1367, https://doi.org/10.1093/eurheartj/ehaa575
Published: 29 August 2020A correction has been published: European Heart Journal, Volume 42, Issue 19, 14 May 2021, Page 1908, https://doi.org/10.1093/eurheartj/ehaa895A correction has been published: European Heart Journal, Volume 42, Issue 19, 14 May 2021, Page 1925, https://doi.org/10.1093/eurheartj/ehab088A correction has been published: European Heart Journal, Volume 42, Issue 23, 14 June 2021, Page 2298, https://doi.org/10.1093/eurheartj/ehab285For the purposes of this document, ECGs are classified into 3 groups: 1) STEMI or equivalent; 2) ischemic ST-segment or T-wave abnormalities; and 3) nonischemic, which includes ECGs interpreted as normal, having nonspecific findings, left ventricular hypertrophy with or without repolarization
ST-T wave changes, and left or right bundle branch block
or paced rhythm (not meeting Sgarbossa9 or modified
Sgarbossa10,11 criteria for MI).5.2.2. Additional ECG Findings Consistent With Acute Coronary Artery Occlusion
The application of STEMI ECG criteria on a standard 12-lead ECG alone will miss a significant minority of patients who have acute coronary occlusion.21 Therefore, the ECG should be closely examined for subtle changes that may represent initial ECG signs of vessel occlusion, such as hyperacute T waves (in the absence of electrolyte imbalances or significant left ventricular hypertrophy) or ST-segment elevation <1 mm, particularly when combined with reciprocal ST-segment depression, as this may represent abnormal coronary blood flow and/or vessel occlusion.21 Concomitant reciprocal ST-segment depression may be visually more evident than the minor ST-segment elevation in such patients.28 If present, these patients should be evaluated for emergent coronary angiography as their outcomes are similar to those with more extensive ST-segment elevation.29
Other ECG findings may also indicate acute coronary artery occlusion. Anterior ST-segment depression (eg, leads V1-V3) may represent acute posterior transmural MI.19 Acute posterior MI can be confirmed by evaluating for the presence of ST-segment elevation on posterior leads19 or emergent echocardiography demonstrating wall motion abnormalities in the posterior and/or inferior territories. Emergent coronary angiography should be performed when there is a high suspicion for acute posterior MI, because delay in reperfusion has been associated with worse outcomes.30 Similarly, de Winter’s sign, suggested by tall, prominent, symmetrical T waves arising from upsloping ST-segment depressions >1 mm in the precordial leads, can be seen in proximal left anterior descending artery occlusion and therefore warrants immediate angiography.18
The identification of acute coronary occlusion among patients with LBBB or ventricular pacing poses particular challenges. The presence of a new LBBB is no longer considered a STEMI equivalent,12 although it is associated with higher risk because most patients with LBBB have underlying cardiac disease, typically CAD or a cardiomyopathy.31 The Sgarbossa criteria (see Table 1) are specific, although not sensitive for acute coronary artery occlusion.9 In a study that included patients with acute left anterior descending artery occlusion, a modification that used an ST/T-wave ratio improved sensitivity from 52% to 91% with similar specificity to the original criteria.10,11 For those meeting the Sgarbossa or modified Sgarbossa criteria, treatment should be similar to those with STEMI. Emergent echocardiography can be performed in cases in which there is an LBBB and suspicion for ischemia/infarction with the caveat that the frequent coexistent cardiomyopathy may make differentiation difficult. Although less well studied, patients with ventricular paced rhythms who have ECG findings that meet Sgarbossa criteria should also be considered high risk and undergo emergent coronary angiography.9,32
5.2.3. ECG Findings Consistent With Ischemia
Wellen’s syndrome is characterized by biphasic or inverted T-wave inversions in the anterior precordial leads in patients whose ischemia has resolved.33 Its presence is associated with proximal left anterior descending artery stenosis and is associated with high rate of subsequent transmural MI.
In patients with ischemic symptoms, ST-segment elevation in lead augmented vector right (with or without elevation in V1) combined with multilead ST-segment depression represents a high-risk ECG finding that is associated with high morbidity and mortality.34 In patients with ischemic symptoms, this often represents diffuse ischemia due to significant stenosis involving the left main and/or 3-vessel disease,35,36 although it can be seen in other non-ACS conditions causing a demand/supply mismatch.37 In approximately 10% of cases, acute coronary occlusion is present.35 Accordingly, management of patients with this ECG pattern must be nuanced. Precipitants of supply/demand mismatch (if present) should be treated. Emergent coronary angiography should be considered in patients with persistent ischemic symptoms or ECG changes after treatment or if there is hemodynamic instability.35
Ischemic ST-segment depression is present in a minority of ACS patients, representing a specific, but not sensitive finding for ACS. In the setting of ischemic symptoms, it should prompt treatment consistent with the 2014 NSTE-ACS guidelines,13 as the majority of patients with ischemic ST-segment depression will be diagnosed with MI.38 T-wave inversion is a less-specific marker of subendocardial ischemia because it can be present in non-ACS conditions. Rather than being indicative of acute ischemia, T-wave inversion can become evident after clinical ischemia resolves. Ischemic T-wave inversion tends to be deeper and new when compared with prior ECGs.
5.2.4. Summary
The ECG is a critical component of the initial assessment and management of ED patients with possible ACS. The ECG should be rapidly assessed for evidence of acute infarction or ischemia, and if present, subsequent care should follow current guidelines for management of acute STEMI12 and NSTE-ACS.13 The ECG should also be examined for subtle changes that are also consistent with ACS as well as for other findings that could suggest a non-ACS cause for their symptoms. Patients who have a nonischemic ECG (as defined previously) are eligible for entering a CDP, and further clinical evaluation should take place as outlined later.
5.3. Hs-cTn Assays
Measurement of cardiac troponin T (cTnT) or cardiac troponin I (cTnI), the gold-standard biomarkers of myocardial injury, is critical for the evaluation of possible ACS in the ED. In the United States, hs-cTn assays are being increasingly adopted because of their ability to detect lower cTn concentrations with improved analytical performance (ie, greater sensitivity and precision) compared with older-generation assays.15
[Please see and review the paragraphs not excerpted for details.]
Concentrations of hs-cTn should be reported in whole numbers without decimal places as nanograms per liter (ng/L), as recommended by the International Federation of Clinical Chemistry and Laboratory Medicine, endorsed in the Fourth Universal Definition of MI,14,39 and adhered to by assay manufacturers. Reporting in ng/L avoids potential confusion related to the use of 3 decimal places and multiple zeroes (for instance, 14 ng/L is preferred over 0.014 ng/mL). 5.3.1. Defining Abnormal Hs-cTn Values
Key questions for the manag.ement of patients with possible ACS in the ED are what constitutes an “abnormal” or an “elevated” hs-cTn value and how to rapidly and reliably differentiate between ACS and the multitude of other potential causes for cTn elevation (see Section 5.7).40 . . . . Similar to population-based studies, minor hs-cTn elevations are also associated with worse outcomes in ED patients who are ruled out for MI.27,45 Based on these findings, no detectable cTn level can be considered entirely “normal.” The higher the cTn value, the more likely it is related to ACS,46, 47, 48 although there is significant overlap among cTn values for type 1 and 2 MI and acute myocardial injury. As a result, hs-cTn values still require interpretation based on the appropriate clinical context. Serial hs-cTn measurements should be performed to confirm the MI diagnosis, and peak values can be used to estimate MI size.49
False-positive and -negative hs-cTn assay results are rare but can occur. False-positive values may be secondary to sample preparation and handling, instrument malfunction, assay interference, and macro troponin complexes.50,51 Although rare, clinicians should be aware that false-negative values can occur as a result of assay interference from ingested substances such as biotin.50 Close collaboration between laboratory medicine professionals and clinicians is necessary to troubleshoot suspected false-positive or -negative values.
5.3.2. 99th Percentile Thresholds
The 2018 Fourth Universal Definition of MI defined biomarker evidence of acute myocardial injury as an acute rise and/or fall in cTn values (≥20% change between serial measurements), with at least 1 cTn value above the assay’s 99th percentile URL.14 Although this definition has undeniable merits, it also has important limitations when applied to the acute evaluation of ED patients with possible MI.
Using sex-specific 99th percentile thresholds only addresses a single determinant of cTn variation in the population and does not account for other important factors that influence the 99th percentile threshold, such as age and renal function.60 Moreover, a focus on the 99th percentile threshold does not capitalize on analytical advances with hs-cTn assays that allow the use of very low values for risk stratification. Newer MI rule-out algorithms have been developed that either de-emphasize or avoid altogether the use of 99th percentile URLs (see Section 5.4).
5.3.3. Summary
Hs-cTnT and hs-cTnI are the preferred biomarkers for the evaluation of patients with possible ACS. In U.S. clinical settings, concentrations are reported when at or above the LoQ; concentrations should be reported as whole numbers in nanograms per liter (ng/L). Detectable values represent a continuum of risk for adverse events; thus, no detectable cTn level can be considered “normal.” Sex-specific 99th percentile cutoffs are endorsed to increase sensitivity for diagnosis of MI among women and specificity among men.
5.4. CDPs Using Hs-cTn Assays
Hs-cTn assays have several intrinsic advantages that have facilitated the development of novel accelerated CDPs designed to shorten the time to exclude (“rule out”) MI. Compared with older-generation assays, hs-cTn assays are both more sensitive and more precise. Increased sensitivity allows exclusion of even minor cTn elevations, permitting rule out of MI with a single blood draw when the hs-cTn value is very low and symptoms have been present for 3 hours or more. Assay precision is particularly important when assessing change over serial measurements at low values. Augmented precision of the hs-cTn assays allows biological changes to be distinguished from assay imprecision (ie, noise). This feature has been capitalized on by algorithms that use the absence of small changes in hs-cTn over 1 to 2 hours to exclude (“rule out”) MI.
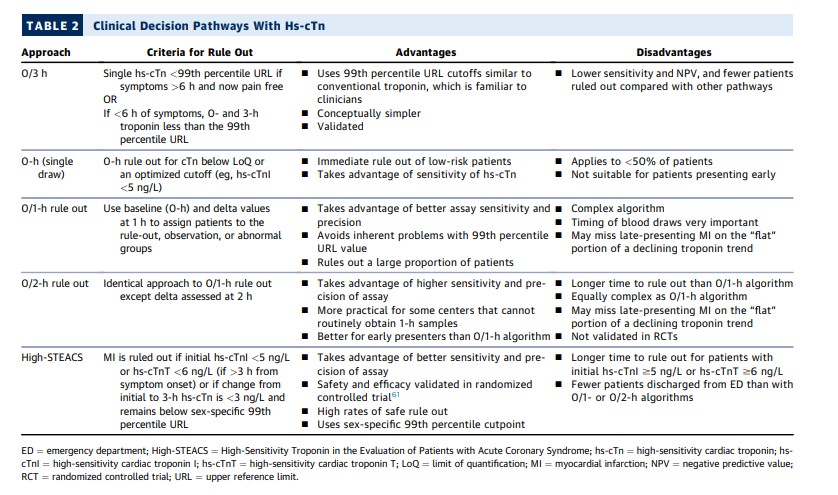
Multiple diagnostic algorithms incorporating hs-cTn have been investigated and implemented. Strengths and weaknesses of several of these approaches are shown in Table 2. The simplest algorithm is conceptually very similar to approaches used in many hospitals with older-generation assays, ruling out patients with an hs-cTn level below the 99th percentile value at 0 and 3 hours, with or without sex-specific 99th percentile URL cutpoints.62 Although this 0/3-hour approach has the advantage of ease of implementation, it fails to capitalize on the advantages of the hs-cTn assays and suffers from all of the limitations of emphasizing the 99th percentile URL value discussed in Section 5.3. More importantly, comparison studies and a recent meta-analysis63 (discussed later) demonstrate that the 0/3-hour approach is inferior to the more innovative 0/1, 0/2, and High-Sensitivity Troponin in the Evaluation of Patients with Acute Coronary Syndrome (High-STEACS) approaches, ruling out a smaller proportion of patients and suffering from higher false-positive diagnosis rates. For this reason, the 0/3-hour approach is not recommended.
5.4.5. Summary
In aggregate, studies performed to date (including real-world implementation studies)95,102,103 demonstrate that the ESC 0/1-hour, 0/2-hour, and High-STEACS CDPs reduce ED length of stay and increase the proportion ruled out and dispositioned home compared with traditional approaches using less-sensitive cTn assays and with the ESC 0/3-hour algorithm using hs-cTn. Implementation of these CDPs is associated with similar clinical outcomes compared with the more traditional approaches that require longer times to rule out MI. With the transition to hs-cTn from older-generation assays, the rate of type 1 MI diagnosis is slightly increased, with greater increases in type 2 MI diagnosis and marked increases in the diagnosis of cardiac injury.68,104 The impact on cardiac testing and coronary angiography has varied slightly between studies, but overall rates do not appear increased102,105,106; however, most have been performed outside of the United States where testing thresholds are different. Importantly, the available data from U.S. studies also showed no significant increase in downstream resource use102,103,105,106, but further data is needed. The absence of improvement in clinical outcomes with implementation of a CDP is expected given their low overall risk.
The ESC 0/1 (or 0/2) and High-STEACS approaches are recommended over simply using the 99th percentile URL value at 0 and 3 hours to rule out MI because direct comparisons demonstrate both greater efficacy (more patients ruled out) and greater safety (fewer missed MIs). Thus, hs-cTn should be implemented in the context of a CDP to achieve maximal performance. Setting objectives and expectations is crucial for successful implementation. The hs-cTn assays diagnose a larger number of patients with type 2 MI and acute and chronic cardiac injury than do approaches using standard assays. Successful implementation of hs-cTn CDPs requires consideration of triage of these patients in ED workflows. The term rule in should be reserved for those in the abnormal group who meet Universal Definition of MI criteria for MI. Careful education is necessary to mitigate untoward consequences, including unnecessary testing and hospitalization for patients without MI (see Section 5.8). The dominant value of the hs-cTn assays is to accelerate chest pain evaluation in the ED, with more patients ruled out faster, allowing more rapid ED discharge, and thus decreasing ED crowding and limiting unnecessary use of resources.