This post consists of links to and excerpts from Resource (1) below, International standardization of diagnostic criteria for microvascular angina ([PubMed Abstract] [Full Text HTML] [Download Full Text PDF from Research Gate]. Int J Cardiol. 2018 Jan 1;250:16-20. doi: 10.1016/j.ijcard.2017.08.068. Epub 2017 Sep 8. The above article has been cited by 14 PubMed Central articles.)
And here are the excerpts:
Introduction
A recent U.S.study in over400,000 individuals undergoing diagnostic coronary angiography for suspected obstructive epicardial coronary disease showed that 59% had either normal coronary arteriograms or non-obstructive (<50% stenosis) coronary artery disease (CAD) [1].Of importance,the arterial coronary tree comprises not only the epicardial arteries,but also smaller arteries and arterioles (<500 μm). The latter feed thecapillaries and represent an important part of the coronary microcir-culation, namely the main site of regulation of myocardial bloodflow. The term coronary microvascular dysfunction (CMD) wasproposed to cover a large number of clinical scenarios characterizedby evidence of a reduced Coronary Flow Reserve (CFR) in the absenceof obstructive epicardial disease [2]. Several studies have demonstrated coronary microvascular dysfunction (CMD) in a large proportion of patients with non-obstructive CAD (~ 30–50%) even afterexclusion of epicardial spasm using provocative testing with acetyl-choline [3,4].
COVADIS, the Coronary Vasomotion Disorders International Study Group, was established to develop standardized criteria for coronary vasomotor disorders thereby facilitating the clinical diagnosis of affected patients and promoting international collabora-tive research endeavors to improve our understanding of these elusive disorders. This paper focuses on the standardization ofcriteria for microvascular angina (MVA) attributable to CMD, inpatients presenting with angina pectoris or ischemic-like symptoms in the absence of flow-limiting CAD (i.e. type 1 CMD according to theoriginal classification proposed by Camici and Crea [2],Table 1). This seems timely as COVADIS has identified several knowledge gaps inthis area, including the need for a better understanding of MVA with regard to: (1) absolute prevalence, (2) optimized diagnostics,(3) efficacy of pharmacologic and other therapeutic strategies and(4) impact on prognosis. To meaningfully address these knowledge gaps clinical registries by COVADIS and other groups have been established and clinical trials are being formulated.
2. Symptoms and clinical manifestations
Similar to patients with obstructive, epicardial CAD, those with MVAdue to CMD may present with typical angina pectoris, atypical symptoms,or angina-equivalent symptoms. Albeit CMD may occur in asymptomaticsubjects, these individuals will be identified only opportunistically giventhe absence of symptoms [5]. . . . It is important to stress the fact that the diagnosis of MVA cannotbe established based on symptoms alone.
3. Objective documentation of myocardial ischemia
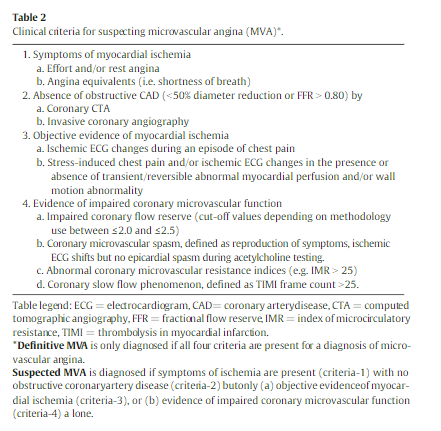
Current guidelines for the diagnosis of stable ischemic heart disease [5,11,12] recommend that symptomatic patients with an intermediate pre-test probability for the presence of obstructive CAD should undergo non-invasive diagnostic testing for detection of myocardial ischemia (Table 2). Objective demonstration of myocardial ischemia should be obtained with rest/stress electrocardiography and/or non-invasive imaging by assessing either myocardial perfusion with single photon
emission computed tomography (SPECT), positron emission tomography (PET) or cardiac magnetic resonance (CMR) or cardiac function with stress echocardiography. During such testing, patients with MVA typically show ST-segment changes and angina, and approximately 20–30% of the patients exhibit transient perfusion defects [13].4. Absence of obstructive/flow-limiting coronary stenoses
The diagnosis of MVA requires – in the first instance – ruling out obstructive/flow limiting CAD as a cause of the ischemic symptoms. The latter is defined as stenoses causing>50% diameter reduction, assessed by conventional angiography or computed tomography angiography [CTA], and/or abnormal (<0.80) fractional flow reserve (FFR). Patients without obstructive CAD may have one of the following on diagnostic coronary angiography: normal or mildly diseased coronary arteries (0% to 30% diameter stenosis), stenosis of “intermediate” severity (30–50%) or diffusely diseased epicardial arteries. In many instances angiography alone may be insufficient to establish whether stenoses b50% are non-obstructive [15]. It is therefore necessary to demonstrate, objectively, that diffuse disease or stenoses of ‘intermediate’ severity are not flow-limiting and FFR should be measured to
identify the hemodynamic relevance of these lesions. However, in some cases microvascular dysfunction may limit microvessel dilation leading to underestimation of physiological stenosis severity by FFR in this setting [16]. . . . CT-FFR is an appealing, emerging noninvasive technology for the assessment of flow-limiting stenoses, but it is probably not as yet sufficiently proven a methodology to be used for this purpose in routine medical practice [17]. In patients with CAD, but with FFR >80, or in those with angiographically normal coronary arteries, the presence of: (1) ischemia-like symptoms and (2) objective evidence of myocardial ischemia, should represent sufficient evidence for the clinician to consider CMD as a likely mechanism responsible for the patient’s symptoms.5. Confirmation of reduced coronary blood flow reserve and/or microvascular spasm causing myocardial ischemia
Currently available techniques do not allow direct visualization of the coronary microcirculation in vivo. Assessment of coronary microcirculatory function can be done invasively and non-invasively using techniques that rely on the functional integrity of the coronary microcirculation. A standard criterion for MVA is the documentation of a reduced CFR [18] and/or the occurrence of microvascular spasm [19](Table 2). The choice
of testing modality relates to availability and expertise, and suspected mechanism. For the assessment of CFR, noninvasive myocardial blood flow measurements using PET [8], assessment of myocardial perfusion with CMR [20] during maximal hyperemia induced by administration of vasodilators, or coronary flow velocity measurements using transthoracic Doppler echocardiography, can also be used [21].Many patients with CMD will undergo invasive coronary angiography and this provides the opportunity for the assessment of flow reserve using invasive techniques normally available in the catheterization laboratory. These include measurement of coronary flow velocity reserve using a Doppler flow wire or of coronary blood flow reserve using a combined pressure/thermodilution wire [25,26]. These techniques have been extensively validated [27] and have been shown to be safe [28].
Coronary microvascular spasm, which is different from focal
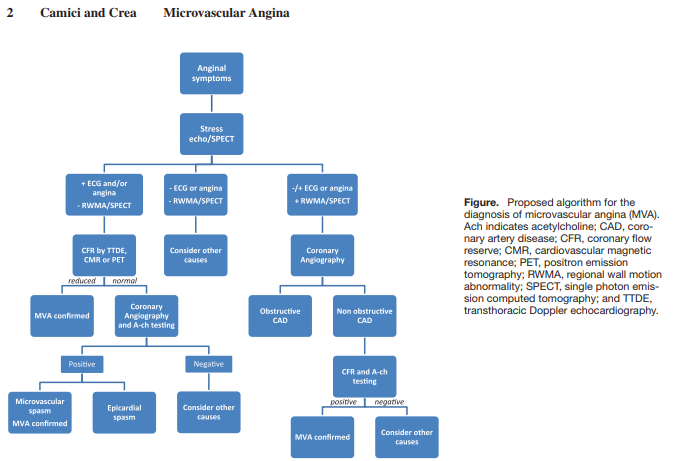
epicardial coronary artery spasm (as seen in Prinzmetal’s variant angina) [32], can be inferred during [invasive] angiographic studies in patients with chest pain despite angiographically unobstructed coronary arteries using intracoronary acetylcholine testing [33]. . . . . An alternative indirect approach evaluates the delayed flow of angiographic contrast, which reflects an increased distal coronary resistance and is known as the “coronary slow flow phenomenon” [35], using the established semi-quantitative TIMI frame count method [36] and diagnostic criteria for the method have been reported previously [37].Camici and Crea have proposed a diagnostic flowchart for the screening of patients with suspected microvascular angina which is based on the use of a combination of noninvasive and invasive tests [38]. [Resource (5) below]
Resources:
(1) International standardization of diagnostic criteria for microvascular angina [PubMed Abstract] [Full Text HTML] [Download Full Text PDF from Research Gate]. Int J Cardiol. 2018 Jan 1;250:16-20. doi: 10.1016/j.ijcard.2017.08.068. Epub 2017 Sep 8.
The above article has been cited by 14 PubMed Central articles.
(2) Diagnosis of Microvascular Angina Using Cardiac Magnetic Resonance [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Am Coll Cardiol. 2018 Mar 6;71(9):969-979. doi: 10.1016/j.jacc.2017.12.046.
The above article has been cited by 8 PubMed Central Articles.
(3) Rationale and design of the Coronary Microvascular Angina Cardiac Magnetic Resonance Imaging (CorCMR) diagnostic study: the CorMicA CMR sub-study [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Open Heart. 2018 Dec 30;5(2):e000924. doi: 10.1136/openhrt-2018-000924. eCollection 2018
(4) How to Diagnose and Manage Angina Without Obstructive Coronary Artery Disease: Lessons from the British Heart Foundation CorMicA Trial [PubMed Abstract] [Full Text] [Full Text PDF]. Interv Cardiol. 2019 May 21;14(2):76-82. doi: 10.15420/icr.2019.04.R1. eCollection 2019 May.
(5) Microvascular Angina: A Women’s Affair? [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Circ Cardiovasc Imaging. 2015 Apr;8(4). pii: e003252. doi: 10.1161/CIRCIMAGING.115.003252.