In this post I link to and excerpt from the chapter, Non-Anion-Gap Metabolic Acidosis, September 17, 2019, of Dr. Farkas’ incredible Internet Book Of Critical Care [Link is to TOC]. And after reviewing the chapter, listen to the 11 minute summary podcast of the chapter.
Dr. Farkas has created this Table of Contents of the chapter and each heading is a direct link to that part of the chapter:
CONTENTS
All that follows are excerpts from the Non-Anion-Gap Metabolic Acidosis Chapter of The Internet Book Of Critical Care [except where noted – material in brackets]:
diagnosis
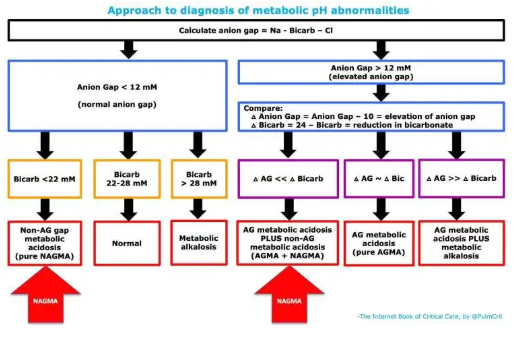
The diagnosis of NAGMA may be made in one of two ways (red arrows above)
- Patient has normal anion gap with metabolic acidosis (bicarbonate < 22 mM).
- Patient has an anion gap metabolic acidosis, but the decrease in bicarbonate is much greater than the elevation in anion gap (indicating the combination of an anion-gap metabolic acidosis plus a non-anion-gap metabolic acidosis).
causes
common causes of NAGMA
- Normal saline infusion
- Resolving diabetic ketoacidosis (discussed further here)
- Gastrointestinal bicarbonate loss
- Diarrhea (especially secretory)
- High-output fistulas, pancreatic/biliary drainage
- Ureteroileostomy or ureterosigmoidostomy
- Renal insufficiency (typically when GFR is between 20-50 ml/min)
- Exogenous acid (e.g. total parenteral nutrition, calcium chloride)
- Chronic hyperventilation (extremely rare)
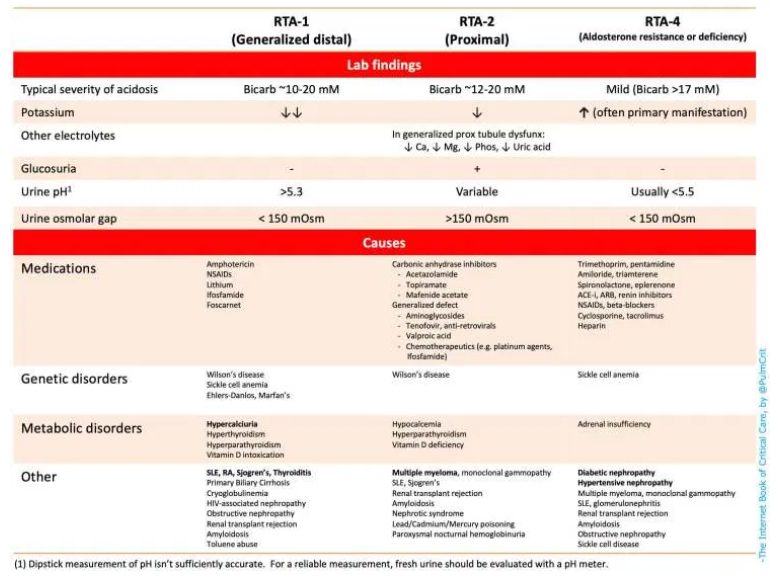
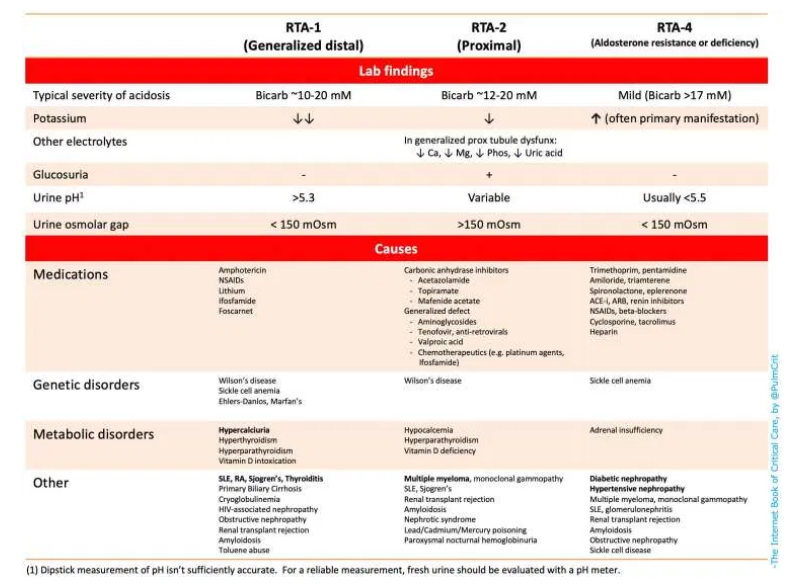
- Renal tubular acidosis (RTA) – Numerous causes shown on the table below 👇
investigation
(1) Is there a clear cause based on clinical history?
- The cause of NAGMA is often fairly clear, based on a review of clinical history and medications.
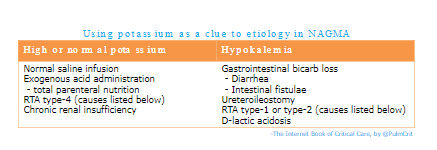
- The potassium level may be used as an early clue to the etiology of NAGMA (table below)(22403272).
- This isn’t 100% accurate, but may help point out the right direction.
- Will be more helpful if the potassium is markedly abnormal. Also, may be more helpful in patients with fewer active medical problems (who are more likely to have a single unifying diagnosis).
- If a likely cause is present, this may simply be treated. Additional diagnostic evaluation may not be needed (unless the patient fails to respond to therapy).
(2) laboratory workup of NAGMA
- Complete electrolytes (including Ca/Mg/Phos)
- Urinalysis
- Urine chemistries:
- Urine sodium, potassium, glucose, urea, osmolarity
- urine pH (Measure accurately with a pH meter)
(3) calculate the urine osmolar gap to diagnose type I or type IV RTA
- Urine osmolar gap may be calculated based on the urine sodium, potassium, glucose, urea, and osmolarity
- Osmolar Gap = Urine osmolarity – 2(sodium + potassium) – Glucose(in mg/dL)/18 – Urea(in mg/dL)/28.
- You can do this using this calculator from QXmD (it’s designed for serum osmolal gap, but it works for urine too).
- The osmolar gap is an indirect measurement of the ammonium (i.e., acid) excreted by the distal nephron. For a patient with acidosis:
- Osmolar gap >150 mOsm indicates appropriate ammonium excretion (excluding RTA type I or IV)
- Osmolar gap <150 mOsm indicates inadequate ammonium excretion (suggests RTA type I or IV)
- Type II (proximal) RTA may secrete normal amounts of ammonium (and thus their urine osmolar gap may be appropriately elevated)(29344509).
- Previously the urinary anion gap has been used for this purpose, but it has numerous limitations (24403272). Specifically, the urinary anion gap may be unreliable due to polyuria, urine pH > 6.5, or the presence of unusual anions such as ketoacids or penicillins (29344509).
(4) which type of RTA?
- The table below summarizes lab findings & causes of RTA.
- Unfortunately, complex ICU patients with numerous electrolyte derangements may defy simple classification.
- Urine pH isn’t well validated in critically ill patients, so this shouldn’t be relied upon (31418093).
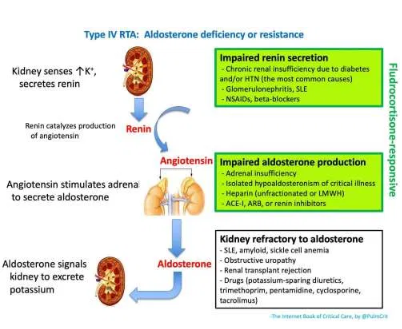
(5) investigation of type IV RTA
- Causes of Type IV RTA are listed above.
- Measurement of renin, aldosterone, and cortisol levels may be helpful (however these will take some time to return).
- Empiric fludrocortisone challenge (e.g. 0.2 mg) might be considered while awaiting laboratory studies. Mineralocorticoid deficiency (green boxes above) is suggested if fludrocortisone induces an increase in the urinary trans-tubular potassium gradient (TTKG) after four hours with a subsequent reduction in serum potassium (24259739, 18216310).
treatment
resolution of underlying cause
- Whenever possible, the underlying cause should be identified and corrected. For example:
- Causative drugs should be discontinued if possible.
- Fludrocortisone may be effective in some patients with type IV RTA due to aldosterone deficiency (discussed right above).
- Unfortunately, treatment of the cause is often insufficient to cause rapid resolution (e.g. in patients with moderate kidney injury). In most cases, further treatment is required as described below.
should NAGMA be treated?
- It is controversial whether NAGMA requires treatment. Although NAGMA frequently correlates with poor outcomes, it’s unclear to what extent it may cause harm.
- Reasonable indications to treat NAGMA with alkali might include:
- The bicarbonate is below ~ 16-18 mEq/L.
- Threshold for treatment may be lower for patients at increased risk of harm from metabolic acidosis (e.g. acute kidney injury or resolving diabetic ketoacidosis).
overview of NAGMA treatment
- NAGMA fundamentally represents an imbalance between sodium chloride and sodium bicarbonate. Treatment therefore may involve addition of sodium bicarbonate and/or removal of sodium chloride. The optimal approach depends on volume status, for example:
- Hypovolemia –> Add sodium bicarbonate.
- Euvolemia –> Add sodium bicarbonate and remove sodium chloride simultaneously. The goal here is to replace bicarbonate with chloride, without shifting the volume status.
- Hypervolemia –> Remove sodium chloride via diuresis.
- This gets a bit more complicated, because different treatments will affect the sodium concentration (which is fundamentally a reflection of the free water balance). For example:
- Hypertonic bicarbonate will increase the sodium concentration.
- Diuresis using only a loop diuretic (e.g. furosemide) will increase the sodium concentration.
- Diuresis using both a loop diuretic plus a thiazide may facilitate removal of sodium chloride without causing hypernatremia (more on this at PulmCrit- Overcoming occult diuretic resistance: Achieving diuresis without dehydration. May 23, 2016 by Dr. Josh Farkas [Note to myself + other readers-Go ahead and review that Pulmcrit post. It is outstanding. I’ll be excerpting it in a seperate post to help fix it in my mind.]).
- The optimal treatment will simultaneously address the NAGMA, the volume status, and the sodium concentration. For example, in a patient with hyponatremia and NAGMA, the use of hypertonic bicarbonate may simultaneously treat both problems.
bicarbonate therapy
- When repleting bicarbonate it is useful to calculate the patient’s bicarbonate deficit, for example with this calculator from MDCalc. This isn’t 100% accurate, but it provides a general concept of the amount of bicarbonate required.
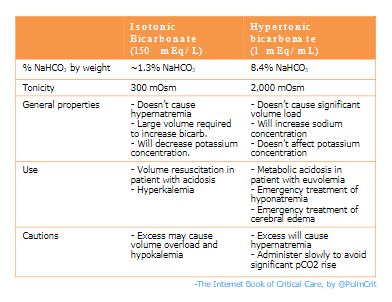
- The use of IV bicarbonate is explored in greater detail here. Some brief comments on various forms:
- (1) Hypertonic bicarbonate
- May be preferred for patients with hyponatremia (will treat both NAGMA and hyponatremia).
- May be used in limited quantities in patients with normonatremia (excessive doses will cause hypernatremia).
- Contraindicated in patients with hypernatremia (will aggravate this).
- (2) Isotonic bicarbonate
- May be preferred for patients with hypovolemia (because it involves a substantial volume load, which will fix both NAGMA and hypovolemia).
- (3) Oral alkali
- This takes longer to work, is overall less effective, and may tend to cause hypernatremia.
- May be useful in cases of mild NAGMA or in patients with ongoing bicarbonate loss (as a maintenance therapy).
- Sodium bicarbonate may be given orally in tablet form (1300 mg = 15 mEq bicarbonate).
- Sodium citrate liquid is an alternative to sodium bicarbonate (citrate is rapidly metabolized to bicarbonate).
- Most formulations (e.g. BICITRA) contains the biologic equivalent of 1 mEq/ml sodium bicarbonate.
- This may be logistically easier to give than sodium bicarbonate tablets (e.g. 30 ml q6hr provides 120 mEq of alkali daily. This is equivalent to sixteen 650-mg tablets of sodium bicarbonate).
- Potassium citrate solution (e.g., POLYCITRA-K) might be useful in NAGMA with hypokalemia. POLYCITRA-K is twice as concentrated as sodium citrate solution (with 2 mEq/ml potassium and generation of 2 mEq alkali per ml).
Pitfalls
- Failure to treat: NAGMA can generally be treated in a supportive fashion (e.g. with IV bicarbonate), even if the precise etiology is unknown. Patients with substantial acidosis should be treated while investigation is ongoing.
- Use of urine anion gap may be misleading. This should generally be avoided, with urine osmolar gap used instead (both of these tests are designed to evaluate for ammonium).