At times, by using noninvasive respiratory support, bi-pap or high flow nasal cannula, we can spare our patient the potential complications of endotracheal entubation and mechanical ventilation.
Dr. Josh Farkas, intensivist and pulmonologist [Link is to Dr. Farkas’ archives, Pulmcrit], has written a number of oustanding posts that clearly explain when and how to use two different types of noninvasive respiratory support [bi-pap, and high flow nasal cannula]. I have listed many of them in Resources below.
As I’ve often written, these posts are my medical study notes. I make excerpts from posts I want to remember to help fix them in my mind [my blog is my medical peripheral brain]. With that in mind, what follows are excerpts from Resource (1), PulmCrit- Mastering the dark arts of BiPAP & HFNC [Link is to Dr. Farkas post – review this rather than my post which is only excerpts],February 12, 2018 by Dr. Farkas:
Skillful use of BiPAP and high-flow nasal cannula (HFNC) can avoid intubation and improve outcomes. However, there isn’t comprehensive evidence about the nitty-gritty details of these techniques. In this post I will use my opinions to fill some gaps in the evidence (1). Noninvasive respiratory support remains more of an art than a science, perhaps a dark art at that.
Summary:
- The role of noninvasive respiratory support is generally to reduce the patient’s work of breathing, thereby avoiding diaphragmatic exhaustion.
- The goal of noninvasive respiratory support isn’t to immediately normalize the ABG.
- Serial evaluation by experienced practitioners is generally far more useful than monitoring ABG values.
- The choice of BiPAP vs. HFNC may be made on the basis of the patient’s diagnosis (e.g. pneumonia vs. heart failure), not the ABG values.
- BiPAP should never be used to “blow off” CO2 in a patient with hypoventilation due to drug intoxication.
Fundamental concepts
Cautions:
- Inadequate monitoring: Techniques described here are designed for an environment with close monitoring and staff available to intubate 24 hours a day.
- Multi-organ failure: Noninvasive respiratory support works best in patients with single-organ respiratory failure.
Pathophysiology of failure: why do patients require intubation for respiratory failure?
To avoid intubation, we must first understand why patients require intubation:
- Hypercapneic encephalopathy (“CO2 narcosis”). These are patients with extremely high CO2 levels (usually pCO2 > 100 mm) causing obtundation.
- Refractory hypoxemia: Inability to oxygenate despite HFNC or BiPAP.
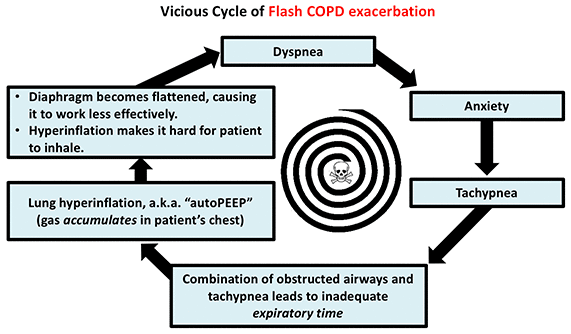
- Respiratory muscle exhaustion: This is the most common reason for intubation, because it represents a final common pathway of respiratory failure. Any type of respiratory failure increases the work of breathing. Eventually, respiratory muscles fatigue and fail. As the diaphragm fails, the ability to cough and clear secretions is lost. This may lead to mucus plugging, which causes acute deterioration.
Given the importance of respiratory muscle fatigue, this post will focus on:
How can we assess respiratory muscle fatigue?
How can BiPAP/HFNC reduce the work of breathing, to prevent muscle fatigue?Goals of therapy
The goal of HFNC or BiPAP is to stabilize the patient, in order to buy time for the underlying disease process to improve. Therefore, my goals are as follows:
- Maintain adequate oxygenation.
- Provide enough ventilatory support so that the patient is comfortable and doesn’t develop respiratory muscle fatigue.
- Ensure that the patient is protecting their airway.
- Serial examination (focused on #1-#3) shows that the patient’s trajectory is either stable or improving (2).
Assessment
For most patients on noninvasive respiratory support, clinical assessment is all that’s needed. This involves three or four pieces:
- Oxygenation: If the patient has a good pulse oximetry waveform, the preferred method is simply to monitor pulse oximetry (ABG is rarely needed to measure oxygenation).
- Work of breathing: The best metric is the respiratory rate. Worsening tachypnea (e.g. respiratory rate >35 b/m) suggests that the patient may eventually tire out. Additional warning signs include retractions, diaphoresis, tripoding, shallow breathing, and an abdominal paradoxical breathing pattern. Asking the patient how they feel is generally useful, but some patients minimize their symptoms.
- Mentation: A patient who is easily arousible and mentating adequately doesn’t have life-threatening hypercapnia (3).
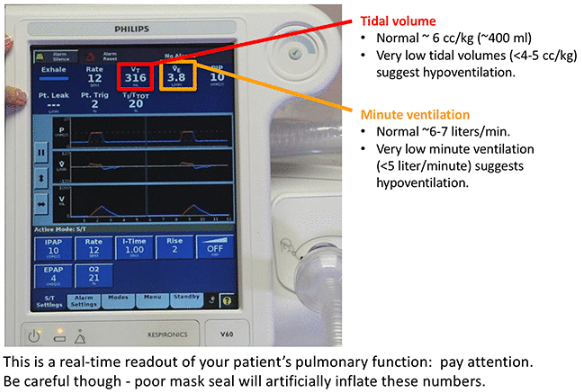
- BiPAP monitor: Low tidal volumes and/or low minute ventilation may suggest hypoventilation (4). Alternatively, adequate tidal volumes and minute ventilation suggest a satisfactory response to BiPAP.
Look at the monitor and the patient, talk to the patient, perhaps try to wake them if they are sleeping. That’s all. An experienced clinician will perform this assessment in about a minute.
The most common problem with assessment is excessive reliance on ABG values. [See PulmCrit- Top 10 reasons pulse oximetry beats ABG for assessing oxygenation November 2, 2016 by Josh Farkas. So much great information including when an ABG is needed].
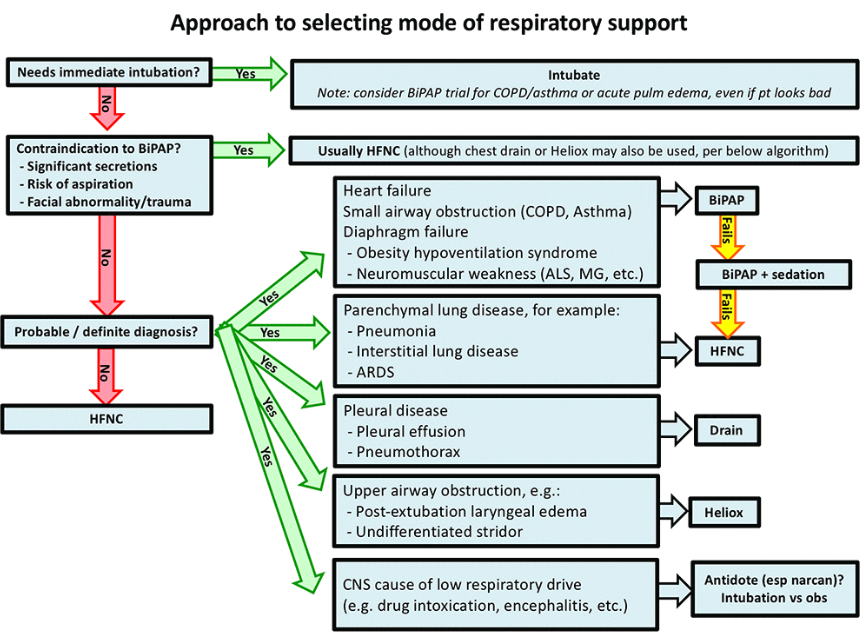
Device selection
Below is my general rubric for noninvasive respiratory support. Let’s walk through it step by step. [In my post I have not included all of Dr. Farkas’ step by step walk through his flow chart. But it needs to be reviewed in Dr. Farkas’ post. ]
Is immediate intubation needed?
The decision to intubate is beyond the scope of this post. However, it’s worth mentioning a few “fake-out” situations, where patients look horrible but usually don’t require intubation:
- Sympathetic crashing acute pulmonary edema (SCAPE) – these patients will often turn around within minutes in response to BiPAP and high-dose nitroglycerine infusion.
- Acute bronchospasm (COPD/Asthma) – may improve dramatically in response to BiPAP, bronchodilation, and sedation (e.g. dexmedetomidine or ketamine).
- Vocal cord dysfunction – patients have true upper airway obstruction, but improve rapidly with sedation or ketamine.
- Pneumothorax, pericardial tamponade, or massive pleural effusion – drain it.
Are there contraindications to full-face BiPAP?
Contraindications include:
- Significant secretions: Positive pressure and the BiPAP mask impair expectoration. Sometimes, it is possible to maintain a patient on BiPAP with occasional breaks on HFNC for secretion clearance (e.g. a COPD patient with mild secretions). However, for a patient with copious secretions, BiPAP is contraindicated. In such cases, BiPAP may initially have excellent results, but eventually mucus plugging occurs with abrupt deterioration.
- Facial trauma, burns, or other anatomic problem with mask seal.
- Risk of aspiration: Aspiration may occur if the patient vomits and is unable to remove the BiPAP mask. Therefore, evaluating aspiration risk requires judging the likelihood of vomiting (e.g. increased with bowel obstruction or pancreatitis) versus the mental status. Note that altered mental status due to hypercapnia isn’t an absolute contraindication to BiPAP.
There are nearly no contraindications to HFNC, so any patient with a contraindication to BiPAP can be treated with HFNC (6). BiPAP with a nasal interface can also be considered here, especially if HFNC isn’t available (7).
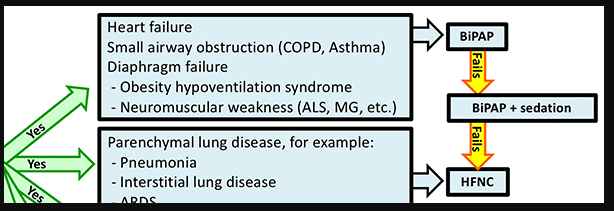
BiPAP-sensitive conditions:
BiPAP has some important advantages compared to HFNC:
- Positive pressure reduces pre-load and after-load on the heart, improving heart failure (this works similar to an ACE-inhibitor – but easier to titrate and no nephrotoxicity).
- BiPAP can provide a greater amount of mechanical support for breathing. This is desirable for patients with respiratory muscle weakness or obesity-hypoventilation syndrome (both conditions involve an imbalance between diaphragmatic strength versus work of breathing).
- For patients with small airway obstruction (e.g. COPD/asthma), BiPAP can provide mechanical support. The expiratory airway pressure (PEEP) may also help stent open airways during exhalation (8).
For patients with these conditions, I will generally make a real effort to use BiPAP. If the patient can’t tolerate BiPAP due to anxiety, it may be worth using sedation to facilitate BiPAP tolerance. Sedation is particularly useful for patients with COPD or asthma, who require a slow respiratory rate in order to exhale properly:
Some brief comments on various sedatives to facilitate BiPAP:
- Dexmedetomidine is very effective and safe if the patient can wait long enough to titrate this up (9). Dexmedetomidine can be up-titrated to induce light sleep, without affecting respiratory drive.
- Ketamine dissociation may be useful up-front, especially in asthma (because it provides bronchodilation). This only provides sedation for 30-60 minutes, so another agent may be needed for ongoing sedation.
- Fentanyl in tiny divided doses may be effective in patients who are very tachypneic, but requires caution and meticulous monitoring (explored further here)(10).
- IV haloperidol or olanzapine may be considered (with the advantage that they don’t suppress the respiratory drive).
- Benzodiazepines have unpredictable effects. They sometimes work, but can also cause confusion or paradoxical agitation.
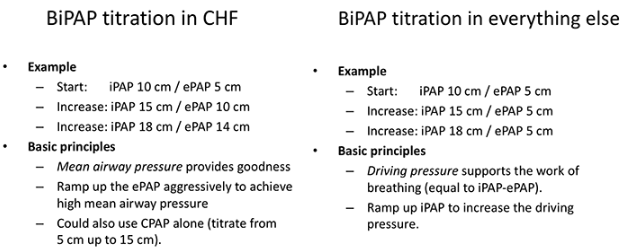
General BiPAP titration schemes are shown below (11):
And remember, as discussed above, that bi-pap is contraindicated in patients with copious secretions:
Significant secretions: Positive pressure and the BiPAP mask impair expectoration. Sometimes, it is possible to maintain a patient on BiPAP with occasional breaks on HFNC for secretion clearance (e.g. a COPD patient with mild secretions). However, for a patient with copious secretions, BiPAP is contraindicated. In such cases, BiPAP may initially have excellent results, but eventually mucus plugging occurs with abrupt deterioration.
And here is when we would use HFNC:
HFNC for patients with parenchymal lung disease (e.g. pneumonia)
Parenchymal lung disease refers to any primary disease of the alveolar tissue itself. The advantages of HFNC here include the following:
- Reduction in work of breathing due to dead space washout
- Preserved ability to cough & clear secretions
- Excellent tolerance, including for extended periods of time (important for patients with interstitial lung disease who may take several days to recover)
- Improved ability to communicate with patients and assess their progress
Compared to heart failure and COPD, parenchymal lung disease is a less BiPAP-responsivephysiology. Studies have found questionable benefit from BiPAP in this situation. The FLORALI trial showed that in a group of patients with hypoxemic respiratory failure (mostly from pneumonia), HFNC was more successful than BiPAP at avoiding intubation and improving mortality.
Treat pleural disease with drainage
Temporize upper airway obstruction with Heliox
And here is when we should avoid both bipap and HFNC:
Avoid BiPAP or HFNC in primary CNS disease
One practice which drives me crazy is the use of BiPAP for treatment of hypercapnia due to drug intoxication. If the patient is so severely intoxicated that they truly need ventilatory support, then they should be intubated (aspiration risk precludes the use of BiPAP here).
Consider HFNC if the diagnosis is unclear
For a patient with unclear diagnosis, either BiPAP or HFNC could be used. My preference is HFNC for the following reasons:
- HFNC facilitates communication, including obtaining additional history.
- BiPAP may make some patients look much worse (due to anxiety) or others look much better (due to treatment of CHF or COPD). This can confuse matters if you don’t know what is going on.
The following is, I think, Dr. Farkas’ most valuable pearl in a post filled with pearls:
Parting shot: the value of empiricism
This post is intended to provide a rational framework, but not a rigid one. Due to individual factors (e.g., mask seal, anxiety), the response of individual patients isn’t entirely predictable. If one device isn’t working, try something else. As long as you are monitoring patients closely to ensure that they are responding adequately, any strategy is acceptable.
Resources:
(1) PulmCrit- Mastering the dark arts of BiPAP & HFNC, February 12, 2018 by Dr. Farkas
(2) High-flow nasal cannula to prevent post-extubation respiratory failure
July 16, 2014 by Josh Farkas
(3) High-flow nasal oxygen therapy in intensive care and anaesthesia [PubMed Abstract] Full Text HTML] [Full Text PDF]. Br J Anaesth. 2018 Jan;120(1):18-27. doi: 10.1016/j.bja.2017.11.010. Epub 2017 Nov 21.
(4) PulmCrit – Optimizing the respiratory drive to avoid failure, December 4, 2017 by Dr. Josh Farkas.
(5) Pneumonia, BiPAP, secretions, and HFNC: New lessons from FLORALI
May 25, 2015 by Dr. Josh Farkas.
(6) PulmCrit- Top 10 reasons pulse oximetry beats ABG for assessing oxygenation
November 2, 2016 by Dr. Josh Farkas.