I’ve just assembled and reviewed in this post the following resources which I think of as Part 2 – My Minicourse Review of the Evaluation Of Chest Pain For Primary Care Clinicians* [meaning me]. As I put it together I reviewed the relevant material from my earlier posts on coronary artery disease.
*See also Part 1 – My Minicourse Review of The Evaluation Of Chest Pain For Primary Care Clinicians
Posted on August 2, 2018 by admin
I first reviewed the abstract of (1) Does This Patient With Chest Pain Have Acute Coronary Syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2015 Nov 10;314(18):1955-65. doi: 10.1001/jama.2015.12735. Here is the abstract:
Abstract
IMPORTANCE:
About 10% of patients with acute chest pain are ultimately diagnosed with acute coronary syndrome (ACS). Early, accurate estimation of the probability of ACS in these patients using the clinical examination could prevent many hospital admissions among low-risk patients and ensure that high-risk patients are promptly treated.OBJECTIVE:
To review systematically the accuracy of the initial history, physical examination, electrocardiogram, and risk scores incorporating these elements with the first cardiac-specific troponin.STUDY SELECTION:
MEDLINE and EMBASE were searched (January 1, 1995-July 31, 2015), along with reference lists from retrieved articles, to identify prospective studies of diagnostic test accuracy among patients admitted to the emergency department with symptoms suggesting ACS.DATA EXTRACTION AND SYNTHESIS:
We identified 2992 unique articles; 58 met inclusion criteria.MAIN OUTCOMES AND MEASURES:
Sensitivity, specificity, and likelihood ratio (LR) of findings for the diagnosis of ACS. The reference standard for ACS was either a final hospital diagnosis of ACS or occurrence of a cardiovascular event within 6 weeks.RESULTS:
The clinical findings and risk factors most suggestive of ACS were prior abnormal stress test (specificity, 96%; LR, 3.1 [95% CI, 2.0-4.7]), peripheral arterial disease (specificity, 97%; LR, 2.7 [95% CI, 1.5-4.8]), and pain radiation to both arms (specificity, 96%; LR, 2.6 [95% CI, 1.8-3.7]). The most useful electrocardiogram findings were ST-segment depression (specificity, 95%; LR, 5.3 [95% CI, 2.1-8.6]) and any evidence of ischemia (specificity, 91%; LR, 3.6 [95% CI,1.6-5.7]). Both the History, Electrocardiogram, Age, Risk Factors, Troponin (HEART) and Thrombolysis in Myocardial Infarction (TIMI) risk scores performed well in diagnosing ACS: LR, 13 (95% CI, 7.0-24) for the high-risk range of the HEART score (7-10) and LR, 6.8 (95% CI, 5.2-8.9) for the high-risk range of the TIMI score (5-7). The most useful for identifying patients less likely to have ACS were the low-risk range HEART score (0-3) (LR, 0.20 [95% CI, 0.13-0.30]), low-risk range TIMI score (0-1) (LR, 0.31 [95% CI, 0.23-0.43]), or low to intermediate risk designation by the Heart Foundation of Australia and Cardiac Society of Australia and New Zealand risk algorithm (LR, 0.24 [95% CI, 0.19-0.31]).CONCLUSIONS AND RELEVANCE:
Among patients with suspected ACS presenting to emergency departments, the initial history, physical examination, and electrocardiogram alone did not confirm or exclude the diagnosis of ACS. Instead, the HEART or TIMI risk scores, which incorporate the first cardiac troponin, provided more diagnostic information.
Next I reviewed the abstract of (2) Outcomes of anatomical versus functional testing for coronary artery disease [PubMed Abstract] [Full Text HTML] [Full Text PDF]. N Engl J Med. 2015 Apr 2;372(14):1291-300. doi: 10.1056/NEJMoa1415516. Epub 2015 Mar 14.
Abstract
BACKGROUND:
Many patients have symptoms suggestive of coronary artery disease (CAD) and are often evaluated with the use of diagnostic testing, although there are limited data from randomized trials to guide care.METHODS:
We randomly assigned 10,003 symptomatic patients to a strategy of initial anatomical testing with the use of coronary computed tomographic angiography (CTA) or to functional testing (exercise electrocardiography, nuclear stress testing, or stress echocardiography). The composite primary end point was death, myocardial infarction, hospitalization for unstable angina, or major procedural complication. Secondary end points included invasive cardiac catheterization that did not show obstructive CAD and radiation exposure.RESULTS:
The mean age of the patients was 60.8±8.3 years, 52.7% were women, and 87.7% had chest pain or dyspnea on exertion. The mean pretest likelihood of obstructive CAD was 53.3±21.4%. Over a median follow-up period of 25 months, a primary end-point event occurred in 164 of 4996 patients in the CTA group (3.3%) and in 151 of 5007 (3.0%) in the functional-testing group (adjusted hazard ratio, 1.04; 95% confidence interval, 0.83 to 1.29; P=0.75). CTA was associated with fewer catheterizations showing no obstructive CAD than was functional testing (3.4% vs. 4.3%, P=0.02), although more patients in the CTA group underwent catheterization within 90 days after randomization (12.2% vs. 8.1%). The median cumulative radiation exposure per patient was lower in the CTA group than in the functional-testing group (10.0 mSv vs. 11.3 mSv), but 32.6% of the patients in the functional-testing group had no exposure, so the overall exposure was higher in the CTA group (mean, 12.0 mSv vs. 10.1 mSv; P<0.001).CONCLUSIONS:
In symptomatic patients with suspected CAD who required noninvasive testing, a strategy of initial CTA, as compared with functional testing, did not improve clinical outcomes over a median follow-up of 2 years. (Funded by the National Heart, Lung, and Blood Institute; PROMISE ClinicalTrials.gov number, NCT01174550.).
And then I reviewed the abstract of (3) A 15-Year Warranty Period for Asymptomatic Individuals Without Coronary Artery Calcium: A Prospective Follow-Up of 9,715 Individuals. JACC Cardiovasc Imaging. 2015 Aug;8(8):900-9. doi: 10.1016/j.jcmg.2015.01.025. Epub 2015 Jul 15.
Abstract
OBJECTIVES:
The aim of this study was to examine the long-term prognosis in asymptomatic individuals with a coronary artery calcium (CAC) score of 0 and its associated warranty period.BACKGROUND:
Emerging evidence supports a CAC score of 0 as a favorable cardiovascular short-to intermediate-term prognostic factor.METHODS:
A total of 9,715 individuals undergoing CAC imaging were stratified by age, Framingham risk score (FRS), and National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) categories and followed for a mean of 14.6 years (range 12.9 to 16.8 years). Cox regression, area under the receiver-operating characteristic curve, and net reclassification information were used to assess all-cause mortality, discrimination, and reclassification of a CAC score of 0 compared with the FRS and NCEP ATP III, respectively. A warranty period was pre-defined as <1% annual mortality rate. Vascular age was estimated by linear regression.RESULTS:
In 4,864 individuals with a baseline CAC score of 0 (mean age, 52.1 ± 10.8 years; 57.9% male), 229 deaths occurred. The warranty period of a CAC score of 0 was almost 15 years for individuals at low and intermediate risk with no significant differences regarding age and sex. A CAC score of 0 was associated with a vascular age of 1, 10, 20, and 30 years less than the chronological age of individuals between 50 and 59, 60 and 69, 70 and 79, and 80 years of age and older, respectively. The CAC score was the strongest predictor of death (hazard ratio: 2.67, 95% confidence interval: 2.29 to 3.11) that enabled discrimination and consistent reclassification beyond the FRS (area under the receiver-operating characteristic curve: 0.71 vs. 0.64, p < 0.001) and NCEP ATP III (area under the receiver-operating characteristic curve: 0.72 vs. 0.64, p < 0.001).CONCLUSIONS:
A CAC score of 0 confers a 15-year warranty period against mortality in individuals at low to intermediate risk that is unaffected by age or sex. Furthermore, in individuals considered at high risk by clinical risk scores, a CAC score of 0 confers better survival than in individuals at low to intermediate risk but with any CAC score.
And then I reviewed my post (4) When is The Evaluation of New or Changed Chest Pain Appropriate in the Office From The 2012 SIHD Guideline
Posted on August 5, 2014 by Tom Wade MD. Here are some excerpts:
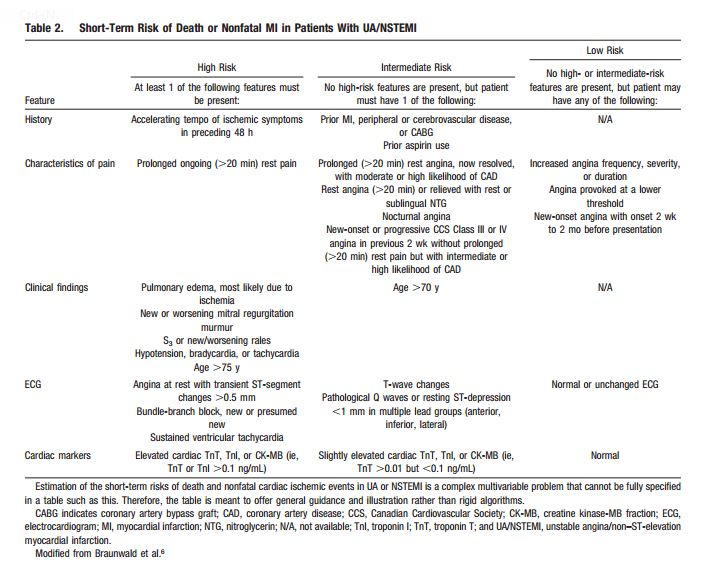
When a patient presents in any clinical encounter with the symptom of new or a change in established chest pain (by phone, in the office, urgent care center, or emergency department), the questions of Table 2 [below] should be asked and answered first. In this way the patient with possible CAD symptoms can be classified into one of the three risk groups [high, intermediate, or low].
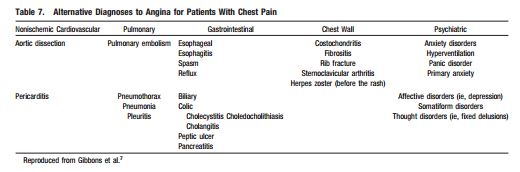
Patients in the first two groups [in the table above] require immediate evaluation in the emergency department. Patients in the third group, can be evaluated using these 2012 ACCF/AHA guidelines. But after asking and answering the questions in Table 2, you also want to consider other possibly life threatening conditions presented in Table 7 [below].
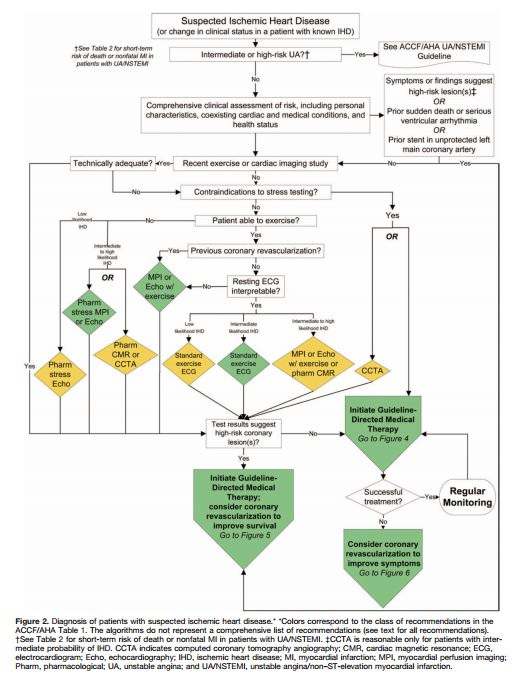
So you’ve reviewed Tables 2 and 7 [above] and you have decided that the patient can be evaluated in the office and that the initial diagnostic evaluation should be noninvasive. And so you proceed by using the Flow Chart in Figure 2 [below].
And then you look at the [the results of the] noninvasive workup [you ordered] to determine the risk of heart attack or death. If there are any high risk findings that suggest that revascularization might reduce his/her risk of death or serious heart damage, then you want to consider coronary angiography. Review [your] w/u looking for the findings in Table 14 [below]. If the patient falls into high or intermediate risk, then serious consideration of coronary angiography is indicated to determine if revascularization can lessen that risk.
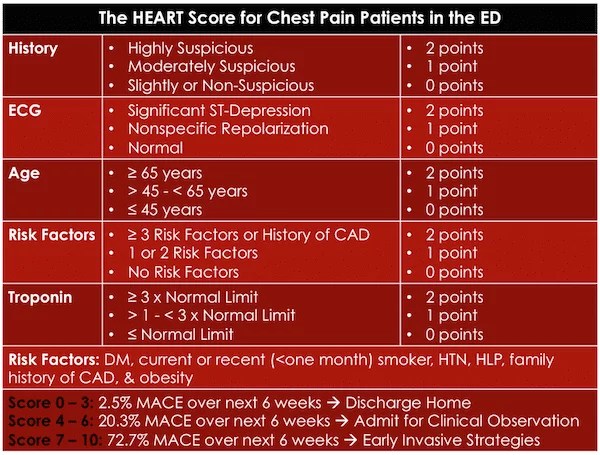
And next I reviewed my post (5) Determining Low Risk Chest Pain Using The Modified Heart Score From Emergency Medicine Cases #64
Posted on March 27, 2016 by Tom Wade MD. Here are some excerpts:
[Here is the original heart score]
This study used a single traditional troponin assay (not the high-sensitivity troponins that are increasingly being used) regardless of the timing of the troponin. Most EM providers would agree that a rate of MACE of 2.5% is not low enough to discharge patients from the ED. However, the Modified HEART Score lowered the risk of MACE to 0.6% by adding high sensitivity Troponins at 0 and 3 hours after arrival at the ED.
The Modified HEART Score
- HEART Score < 3, and
- Negative high sensitivity troponins at 0 and 3 hours
The rate of MACE in these patients was 0.6% within 6 weeks.
A subsequent study in March 2015 using the modified HEART score showed significant decreases in objective cardiac testing and median length of stay and increases in early discharge rates while maintaining a zero MACE rate at 30 days.
And next I reviewed my post (6) I reviewed my post Imaging Of Acute Coronary Syndrome: “Appropriate Utilization of Cardiovascular Imaging in Emergency Department Patients With Chest Pain” Posted on November 27, 2017. The following are all excerpts from the ACR’s article on appropriate imaging criteria.
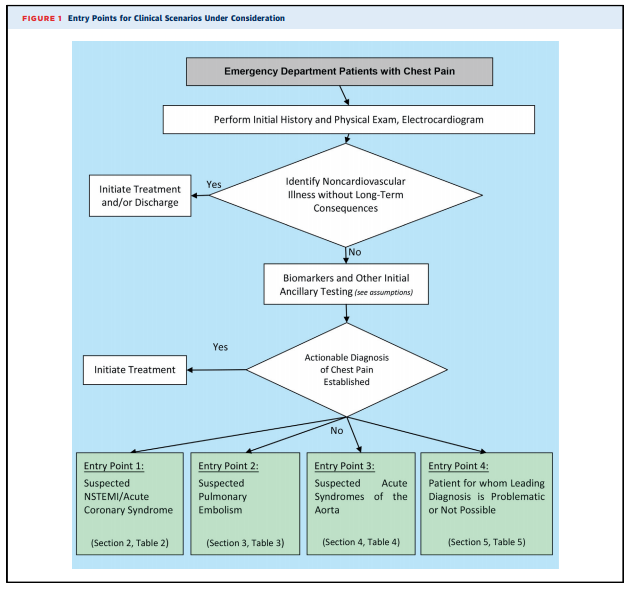
Because the charge of the writing group is to describe common clinical scenarios seen in contemporary practice, the document is organized with respect to diagnostic algorithms from four key clinical entry points that direct imaging (see Figure 1):
- Suspected non–ST-segment elevation ACS (clinical scenarios 1-10)
- Suspected PE (clinical scenarios 11-15)
- Suspected acute syndrome of the aorta (clinical scenarios 16-18)
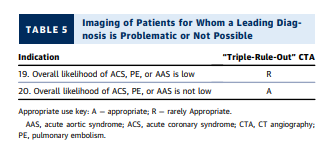
- Patients for whom a leading diagnosis is problematic or not possible (clinical scenarios 19 and 20).
There are three groups of appropriateness ratings:
- Appropriate – rating 7, 8, or 9
- May be appropriate – rating 4, 5, or 6
- Rarely appropriate – rating 1, 2, or 3
Entry Criteria Into Algorithms
1. All adult patients presenting to EDs with potential CP syndromes will undergo evaluations that generally include history and physical examination, immediate electrocardiography (ECG) to identify or exclude STsegment elevation myocardial infarction (STEMI), and cardiac and/or pulmonary biomarker analysis (troponin and/or D-dimer) (Figure 1). Some patients will be diagnosed with noncardiovascular illnesses that exclude ACS, PE, and AAS, and in general, no imaging is required. Patients with evidence of STEMI on initial ECG or initial biomarkers and/or ECG clearly consistent with ACS or NSTEMI are admitted and treated according to evidence-based guidelines. These patients are, in general, not the subjects of this document.
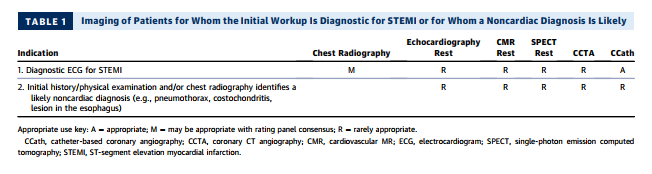
2. Table 1 evaluates the role of imaging in the process of the initial workup, with two common scenarios that include patients for whom ECG is diagnostic for STEMI and patients for whom an alternative, noncardiac diagnosis is likely.
3. After the initial evaluation, it is assumed that the physician will be able to clinically risk-stratify the majority of those remaining patients into one of the three suspected diagnoses of concern: ACS (Section 2), PE (Section 3), and AAS (Section 4). Section 5 includes the minority of patients for whom a leading diagnosis is not possible. Sections 2 through 5 assume that the initial workup and ancillary testing, including cardiac and/or pulmonary biomarkers, are completed (Figure 1).
4. Some patients who enter the clinical scenarios and undergo imaging studies will have inconclusive data to confirm or exclude a leading diagnosis after imaging. Although ratings for Sections 2 through 5 may have more than one imaging study that may be considered appropriate, this document does not specifically address the appropriate use of a second imaging study. The writing group acknowledges that although such patients can present a diagnostic dilemma, there are limited or no data on which to establish appropriate use criteria for the second study, particularly because findings from the first study may influence the best choice for subsequent imaging.
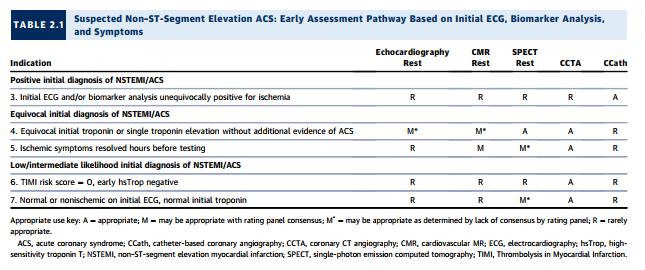
5. One-third of patients with confirmed acute myocardial infarction (AMI) will not have typical CP; Section 2 includes those patients.
6. Imaging in the ED alone, or during evaluation in an observation unit, is considered in this document. Some patients may be candidates for outpatient referrals for follow-up imaging in lower intensity settings. The clinical scenarios in this document in general do not cover these referrals, nor does this document include imaging for patients who do not present to the ED.
7. Miniaturization of ultrasound technology has enabled the use of focused cardiac ultrasound (FOCUS), or bedside ultrasonography performed by the emergency medicine physician, using highly portable equipment that lends itself well to use in an ED setting when a rapid evaluation is required. [There is an extensive discussion in part 7 of the appropriate use of this modality but it was not considered by the rating panel. See Section 7 in the text for details.]
Comorbidities and Contraindications
Patients under consideration for rating among imaging tests do not have specific comorbidities or contraindications as noted below.
1. Unless otherwise stated, the following absolute or relative contraindications that would preclude certain types of imaging are assumed not to be present: claustrophobia, pregnancy, iodine allergy, renal dysfunction, and high resting heart rate.
2. Imaging studies that deliver ionizing radiation are, in general, relatively contraindicated during pregnancy.
3. Gadolinium-enhanced MRI is, in general, not performed in patients who are pregnant.
Clinical Scenario 6: TIMI Risk Score = 0, Early hsTrop Negative
As noted under “Testing Considerations,” although high sensitivity troponins (13) are, at the time of rating [meaning at the time of this article], not approved for use in the United States, they are increasingly used outside the United States. Moreover, an emerging body of literature suggests that incorporating these biomarkers can identify a group of patients already at very low clinical risk whose ACS prevalence and event rate are very low. Conceptually, in such a setting, no further testing may be considered, as the yield would likely be low. The rating panel has considered CCTA appropriate in this setting, as some of the extant trials of CCTA versus standard-of-care evaluation have generally included relatively low-risk populations. In one study, there were no cardiac deaths, and only 1% of patients had MIs within 30 days (38). In this population, CCTA was rated as appropriate, and all other imaging modalities were rated as rarely appropriate.
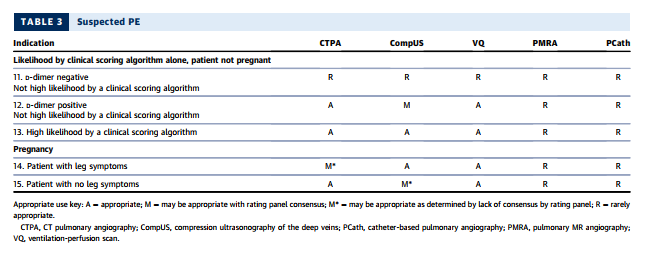
Multidetector-row CT scanners rapidly image the entire chest with high spatial resolution (121–123,125,126), and extant guidelines (120,127) have demonstrated CTPA as a useful diagnostic strategy to exclude or confirm the presence of a filling defect in a patient for whom there is clinical suspicion for PE. In a meta-analysis of 3,500 patients undergoing CTPA and followed for at least three months, the overall NPV of CT was 99.4% (128). A validated outcome strategy is D-dimer testing followed by CTPA for patients with abnormally elevated D-dimer levels. Using this strategy, only 1.5% of patients with negative findings developed DVT or PE during 3-month follow-up (129). A systematic review of management outcome studies showed that patients with low or moderate pretest probability and normal D-dimer levels had a very low 3-month thromboembolism rate (130). CTPA also identifies other pulmonary diseases, including pneumonia, atelectasis, pneumothorax, and pleural effusion, that might not be well visualized on chest radiography.
The latest generation scanners can image thrombus in sixth-order vessels (131). These thrombi are so tiny that their clinical significance is uncertain (132). The presence of right-heart strain is a poor prognostic factor for patients with PE; therefore, the interpreter should compare the size of the right ventricle with that of the left ventricle in positive cases (133), as a normal right ventricle’s diameter is smaller than that of the left ventricle.
CTPA using rapid imaging protocols can include additional scanning to identify DVT in the subclavian veins and other major upper extremity veins that might contain thrombi and serve as the source of PE. Although protocols have been developed and tested for imaging the venous system in the abdomen, pelvis, thighs, and knees for pelvic vein thrombosis and proximal leg DVT (134), CT venography is not routinely used at the time of pulmonary angiography, as it increases radiation exposure and rarely changes clinical management (131,135,136). Combined CTPA and CT venography were not considered by the rating panel.
The latest generation scanners can image thrombus in sixth-order vessels (131). These thrombi are so tiny that their clinical significance is uncertain (132). The presence of right-heart strain is a poor prognostic factor for patients with PE; therefore, the interpreter should compare the size of the right ventricle with that of the left ventricle in positive cases (133), as a normal right ventricle’s diameter is smaller than that of the left ventricle.
CTPA using rapid imaging protocols can include additional scanning to identify DVT in the subclavian veins and other major upper extremity veins that might contain thrombi and serve as the source of PE. Although protocols have been developed and tested for imaging the venous system in the abdomen, pelvis, thighs, and knees for pelvic vein thrombosis and proximal leg DVT (134), CT venography is not routinely used at the time of pulmonary angiography, as it increases radiation exposure and rarely changes clinical management (131,135,136). Combined CTPA and CT venography were not considered by the rating panel.
SUSPECTED PE IN PREGNANCY
See the article, pp. 869 + 870. ACR/ACC/AHA/AATS/ACEP/ASNC/NASCI/SAEM/SCCT/SCMR/SCPC/SNMMI/STR/STS Appropriate Utilization of Cardiovascular Imaging in Emergency Department Patients With Chest Pain: A Joint Document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria Task Force [PubMed Citation] [Full Text HTML] [Download Full Text PDF]. J Am Coll Cardiol. 2016 Feb 23;67(7):853-79. doi: 10.1016/j.jacc.2015.09.011. Epub 2016 Jan 22.
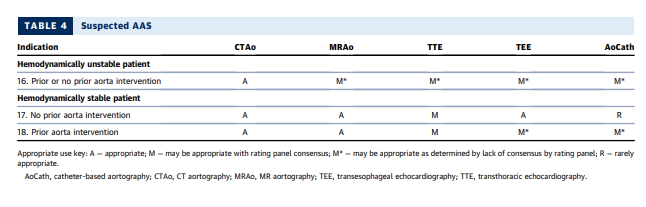
SECTION 4: IMAGING OF PATIENTS WITH SUSPECTED ACUTE SYNDROMES OF THE AORTA
Resources
(1) Does This Patient With Chest Pain Have Acute Coronary Syndrome?: The Rational Clinical Examination Systematic Review [PubMed Abstract]. JAMA. 2015 Nov 10;314(18):1955-65. doi: 10.1001/jama.2015.12735.
(2) Outcomes of anatomical versus functional testing for coronary artery disease [PubMed Abstract] [Full Text HTML] [Full Text PDF]. N Engl J Med. 2015 Apr 2;372(14):1291-300. doi: 10.1056/NEJMoa1415516. Epub 2015 Mar 14.
(3) A 15-Year Warranty Period for Asymptomatic Individuals Without Coronary Artery Calcium: A Prospective Follow-Up of 9,715 Individuals [PubMed Abstract] [Full Text HTML] [Full Text PDF]. JACC Cardiovasc Imaging. 2015 Aug;8(8):900-9. doi: 10.1016/j.jcmg.2015.01.025. Epub 2015 Jul 15.
(4) When is The Evaluation of New or Changed Chest Pain Appropriate in the Office From The 2012 SIHD Guideline Posted on August 5, 2014 by Tom Wade MD
(5) Determining Low Risk Chest Pain Using The Modified Heart Score From Emergency Medicine Cases #64 Posted on March 27, 2016 by Tom Wade MD
(6) Imaging Of Acute Coronary Syndrome: “Appropriate Utilization of Cardiovascular Imaging in Emergency Department Patients With Chest Pain” Posted on November 27, 2017