Note to myself and my readers: I only post excerpts from articles because doing so helps me remember the important points [spaced repetition]. Readers should download the complete article [links below] and review that.
In this post I link to and excerpt from Section 6 Angina without obstructive disease in the epicardial coronary arteries from 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) [PubMed Abstract] [Full Text HTML] [Full Text PDF]. European Heart Journal, Volume 41, Issue 3, 14 January 2020, Pages 407–477.
Here are the links to the above guidelines:
Article Contents
All that follows is from
Section 6 Angina without obstructive disease in the epicardial coronary arteries
In clinical practice, a marked discrepancy between findings regarding coronary anatomy, the presence of symptoms, and the results of non-invasive tests frequently occurs.13 These patients deserve attention, as angina and non-obstructive disease are associated with an increased risk of adverse clinical events.14 Low diagnostic yield of ICA can be explained by the presence of: (i) stenoses with mild or moderate angiographic severity, or diffuse coronary narrowing, with underestimated functional significance identified by ICA; (ii) disorders affecting the microcirculatory domain that escape the resolution of angiographic techniques; and (iii) dynamic stenoses of epicardial vessels caused by coronary spasm or intramyocardial bridges that are not evident during CTA or ICA. Intracoronary pressure measurements are useful in circumventing the first of these scenarios. For diagnostic workup, patients with angina and/or myocardial ischaemia showing coronary stenoses with non-ischaemic FFR or iwFR values may also be labelled as having non-obstructive epicardial disease.
The presence of clear-cut anginal symptoms and abnormal non-invasive tests in patients with non-obstructed epicardial vessels should lead to the suspicion of a non-obstructive cause of ischaemia. Quite often, and mainly as a result of persistence of symptoms, patients with angina and no obstructive disease undergo multiple diagnostic tests, including repeated coronary CTA or ICA, that contribute to increased healthcare costs.409 Because diagnostic pathways to investigate microcirculatory or vasomotor coronary disorders are often not implemented, a final diagnosis supported by objective evidence is seldom reached. Owing to this, patient dismay and depression are not rare in this clinical population.410,411 Of note, the use of a structured, systematic approach to explore microcirculatory and vasomotor disorders in patients with non-obstructive CAD, as delineated below, has been shown to increase diagnostic yield.412,413 Furthermore, an RCT, which reported in 2018, found that in patients with non-obstructive coronary disease, tailored treatment guided by the results of intracoronary testing [coronary flow reserve (CFR), microcirculatory resistance, and acetylcholine testing] resulted in a significant reduction of anginal symptoms, compared with conventional, non-guided medical treatment.414
6.1 Microvascular angina
Patients with microvascular angina typically have exercise-related angina, evidence of ischaemia in non-invasive tests, and either no stenoses or mild-to-moderate stenoses (40–60%), revealed by ICA or CTA, that are deemed functionally non-relevant.415 Given the similarity of angina symptoms, a microvascular origin of angina is typically suspected, after excluding obstructive epicardial coronary stenoses, during diagnostic workup of patients with suspected myocardial ischaemia. Regional LV wall motion abnormalities rarely develop during exercise or stress in patients with microvascular angina.412,416 Some patients may also have a mixed pattern of angina, with occasional episodes at rest, particularly associated with exposure to cold.
Secondary microvascular angina, in the absence of epicardial obstruction, may result from cardiac or systemic conditions, including those that cause LV hypertrophy (such as hypertrophic cardiomyopathy, aortic stenosis, and hypertensive heart disease) or inflammation (such as myocarditis or vasculitis).417
6.1.1 Risk stratification
The presence of microcirculatory dysfunction in patients with CCS entails a worse prognosis than originally thought, probably because most recent evidence has been based on follow-up of patients in whom abnormalities in the microcirculation have been objectively documented with invasive or non-invasive techniques.418–423
Microcirculatory dysfunction precedes the development of epicardial lesions, particularly in women,419 and is associated with impaired outcomes. Among patients with diabetes undergoing diagnostic workup, those without obstructive epicardial disease but with an abnormal CFR have similarly poor long-term prognosis as those with obstructive epicardial disease.421 In patients with non-significant coronary stenoses by FFR, the presence of abnormal CFR is associated with an excess of events in the long-term,418,422,423 particularly when the index of microcirculatory resistance (IMR)* is also abnormal.422
*Index of Microcirculatory Resistance: The Basics [accessed 6-20-2019]. William F. Fearon, MD, Associate Professor of Medicine. Director, Interventional Cardiology
Stanford University Medical Center
6.1.2 Diagnosis
The possibility of a microcirculatory origin of angina should be considered in patients with clear-cut angina, abnormal non-invasive functional tests, and coronary vessels that are either normal or have mild stenosis deemed functionally non-significant on ICA or CTA. One of the challenges in performing a comprehensive assessment of microvascular function is testing the two main mechanisms of dysfunction separately: impaired microcirculatory conductance and arteriolar dysregulation.424–426 Yet, outlining which of these two pathways is affected is critically relevant in setting medical treatment to relieve patient symptoms.414
Impaired microcirculatory conductance can be diagnosed by measuring CFR or minimal microcirculatory resistance (the inverse of conductance). CFR can be measured non-invasively with transthoracic Doppler echocardiography [by imaging left anterior descending (LAD) flow],427 magnetic resonance imaging (myocardial perfusion index),428–430 or PET.431 Microcirculatory resistance can be measured in the catheterization laboratory by combining intracoronary pressure with thermodilution-based data (to calculate the IMR) or Doppler flow velocity (to calculate hyperaemic microvascular resistance or HMR).432,433 Both intracoronary thermodilution and Doppler allow the calculation of CFR. For decision-making purposes, values of IMR ≥25 units or CFR <2.0 are indicative of abnormal microcirculatory function.414 Both CFR and IMR are typically measured while using intravenous vasodilators, such as adenosine or regadenoson.
In contrast, the diagnosis of arteriolar dysregulation requires the assessment of endothelial function in the coronary microcirculation by selective intracoronary acetylcholine infusion (see section 6.5). In the presence of dysfunctional vascular endothelium or abnormal function of smooth muscle cells, acetylcholine (an endothelium-dependent vasodilator that also acts directly on smooth muscle cells) triggers paradoxical arteriolar vasoconstriction.434 Thus, in patients with microvascular angina and arteriolar dysregulation, acetylcholine challenge is likely to trigger microvascular spasm. This arteriolar response to acetylcholine causes anginal symptoms with or without concomitant ischaemic ECG changes, and a decrease in coronary blood flow velocity if concomitant Doppler measurements are performed. Peripheral pulse tonometry during reactive hyperaemia may also reveal abnormal systemic endothelial function in patients with angina and non-obstructive CAD.435
6.1.3 Treatment
Treatment of microvascular angina should address the dominant mechanism of microcirculatory dysfunction. In patients with abnormal CFR <2.0 or IMR ≥25 units, and a negative acetylcholine provocation test, beta-blockers, ACE inhibitors, and statins, along with lifestyle changes and weight loss, are indicated.436,437 Patients developing ECG changes and angina in response to acetylcholine testing but without severe epicardial vasoconstriction (all suggestive of microvascular spasm) may be treated like vasospastic angina patients. The effectiveness of a tailored treatment strategy was investigated in the CorMiCa trial, which randomized 151 patients to a stratified medical treatment (based on the results of CFR, IMR, and acetylcholine testing) vs. a standard-care group (including a sham interventional diagnostic procedure). At 1 year, there was a significant difference in angina scores favouring patients assigned to the stratified medical treatment arm.414
6.2 Vasospastic angina
Vasospastic angina should be suspected in patients with anginal symptoms occurring predominantly at rest, with maintained effort tolerance. The likelihood of vasospastic angina increases when attacks follow a circadian pattern, with more episodes at night and in the early morning hours. Patients are frequently younger and have fewer cardiovascular risk factors than patients with effort angina, except for cigarette smoking.442 Coronary vasospasm should be also suspected in patients with patent coronary stents and persistent angina.443,444
6.2.1 Diagnosis
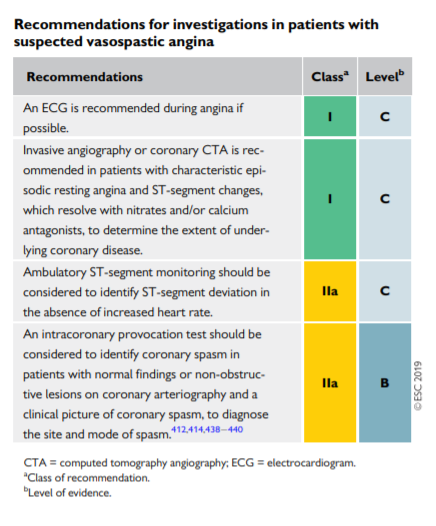
The diagnosis of vasospastic angina is based on detecting transient ischaemic ST-segment changes during an angina attack (usually at rest). Patients with Prinzmetal angina represent a special subset in whom resting angina is accompanied by transient ST-segment elevation.442,445 These ECG changes correlate with proximal vessel occlusion and diffuse, distal subocclusive narrowing of epicardial vessels. As most attacks of vasospastic angina are self-limiting, documentation of these ECG changes is challenging. Ambulatory ECG monitoring, preferably with 12 lead recording, may be helpful in patients in whom vasospastic angina is suspected. The occurrence of ST-segment shifts at normal heart rate supports the likelihood of myocardial ischaemia caused by spasm. Extended Holter monitoring (for >1 week) may be required for successful documentation of transient ST-segment changes in these patients. Ambulatory ECG monitoring may also be used to assess the results of medical therapy in controlling the frequency of vasospastic events.
In patients with suspected vasospastic angina and documented ECG changes, CTA or ICA is indicated to rule-out the presence of fixed coronary stenosis. Angiographic documentation of coronary spasm requires the use of a provocation test in the catheterization laboratory. Given the low sensitivity of hyperventilation and the cold pressor test, intracoronary administration of acetylcholine or ergonovine during ICA are the preferred provocation tests.442 Both pharmacological agents are safe, provided that they are selectively infused into the left or right coronary artery, and that triggered spasm is readily controlled with intracoronary nitrates. A low percentage of patients may develop ventricular tachycardia/ventricular fibrillation or bradyarrhythmias during the provocation test (3.2 and 2.7%, respectively), similar to that reported during spontaneous spasm attacks (7%).446 Intravenous administration of ergonovine for non-invasive tests should be discouraged due to the risk of triggering prolonged spasm in multiple vessels, which may be very difficult to manage and can be fatal.447
A provocation test for coronary spasm is considered positive when it triggers: (i) anginal symptoms, (ii) ischaemic ECG changes, and (iii) severe vasoconstriction of the epicardial vessel. Should the test fail in triggering all three components, it should be considered equivocal.442 The development of angina in response to acetylcholine injections in the absence of angiographically evident spasm, with or without accompanying ST-segment changes, may indicate microvascular spasm and is seen frequently in patients presenting with microvascular angina.445
6.2.2 Treatment
In patients with epicardial or microcirculatory vasomotor disorders, CCBs and long-acting nitrates constitute the treatment of choice, in addition to the control of cardiovascular risk factors and lifestyle changes.437,445 Nifedipine has been shown to be effective in reducing coronary spasm associated with stent implantation.444