In this post I link to and excerpt from 2017 ACC Expert Consensus Decision
Pathway for Optimization of Heart Failure Treatment: Answers to
10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction.
A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways [PubMed Abstract]* [Full Text HTML] [Full Text PDF]. JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY VOL. 71, NO. 2, 2018.
*This PubMed Abstract has links to similar articles. [For the list, please see my post Additional Articles On Heart Failure Treatment From The AHA and ACC
Posted on July 7, 2020 by Tom Wade MD
*This PubMed Abstract has links to articles that cite the above article: See all “Cited by” articles
From the article:
Although some of the recommendations may be
relevant to patients hospitalized with acute HF, this
document mainly deals with the management of
patients with chronic ambulatory HFrEF. [Emphasis Added]
And here are excerpts from 2017 ACC Expert Consensus Decision
Pathway for Optimization of Heart Failure Treatment: Answers to
10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction:
Abstract
The care of patients with HF is more involved than ever. Current care for the patient with HF with reduced ejection fraction (EF) includes no fewer than 7 evidence-based medications, 3 evidence-based device strategies, and a number of recommend processes of care. The opportunity to change the natural history of HF with reduced EF has never been better, but with more choices comes greater complexity.
1. INTRODUCTION
HF exists in several phenotypes, in part reflected by
differences in left ventricular ejection fraction (LVEF).
These include heart failure with reduced ejection fraction
(HFrEF), HF with preserved EF, as well as HF with
improved EF. Although the evidence base for the treatment of HFrEF has expanded substantially, much work
remains for the other forms of HF. New therapies for HF
with preserved EF are under exploration, and the evidence
base addressing HF with improved EF is just emerging.The purpose of this document is to complement
the 2017 ACC/AHA/HFSA Focused Update of the 2013
ACC/AHA Guideline for the Management of Heart Failure (1) by addressing new medical therapies, prevention, and comorbidities relevant to HFrEF for which data are available.Ten Pivotal Issues in HFrEF
1. How to initiate, add, or switch therapy to new
evidence-based guideline-directed treatments for
HFrEF.
2. How to achieve optimal therapy given multiple drugs
for HF including augmented clinical assessment that
may trigger additional changes in guideline-directed
therapy (e.g., imaging data, biomarkers, and filling
pressures).
3. When to refer to an HF specialist.
4. How to address challenges of care coordination.
5. How to improve adherence.
6. What is needed in specific patient cohorts: African
Americans, the frail, and older adults.
7. How to manage your patients’ cost of care for HF.
8. How to manage the increasing complexity of HF.
9. How to manage common comorbidities.
10. How to integrate palliative care and transition to
hospice care.2. METHODS
[See this section in the article.]
3. ASSUMPTIONS AND DEFINITIONS
General Clinical Assumptions
1. Although many topics are generalizable to all
patients with HF, the focus of this effort, including
pathway recommendations, only applies to patients
with HFrEF.
2. Although some of the recommendations may be
relevant to patients hospitalized with acute HF, this
document mainly deals with the management of
patients with chronic ambulatory HFrEF. 3. The expert consensus writing committee endorses
the evidence-based approaches to HF therapy and
management enumerated in the 2013 ACC/AHA Guideline for the Management of Heart Failure (2) and the
2016 and 2017 ACC/AHA/HFSA focused updates (1,3).
4. These algorithms assume the clinician will seek input
as needed from a pharmacist, cardiologist, HF
specialist and/or disease management program, and
other relevant medical specialist (e.g., endocrinologist
or nephrologist) to guide clinical management, and will
consider patient preference in all clinical decisionmaking.
5. These algorithms are based on best available data; all
clinical decisions should be governed by judgment and
influenced by discussions with the patient about
treatment preferences.
6. At any point in time, these suggestions and algorithms
may be superseded by new data.Definitions
HFrEF: Clinical diagnosis of HF and LVEF ≤40%.
New York Heart Association (NYHA) functional
classification:
- Class I: No limitation of physical activity. Ordinary
physical activity does not cause symptoms of HF.- Class II: Slight limitation of physical activity.
Comfortable at rest, but ordinary physical activity results in symptoms of HF.- Class III: Marked limitation of physical activity.
Comfortable at rest, but less than ordinary activity
causes symptoms of HF.- n Class IV: Unable to perform any physical activity
without symptoms of HF, or symptoms of HF at rest.GDMT: Guideline-directed medical therapy.
Optimal therapy: Treatment provided at either the target
or the highest-tolerated dose for a given patient.
Target dose: Doses targeted in clinical trials.
ACC/AHA Stages of HF:
- Stage A: At high risk for HF but without structural heart disease or symptoms of HF.
- Stage B: Structural heart disease but without signs or
symptoms of HF.- Stage C: Structural heart disease with prior or current symptoms of HF.
- Stage D: Refractory HF requiring specialized interventions.
4. PATHWAY SUMMARY GRAPHIC
Figure 1 summarizes the 2017 ACC Expert Consensus
Decision Pathway for Optimization of Heart Failure
Treatment: Answers to 10 Pivotal Issues About Heart
Failure With Reduced Ejection Fraction.5. DESCRIPTION AND RATIONALE:
ANSWERS TO 10 PIVOTAL ISSUES IN HF[Note to myself: Review the answers to the 10 Pivotal Issues In Heart Failure in the article.]
ANSWERS TO 10 PIVOTAL ISSUES IN HF …….. 205
1. How to Initiate, Add, or Switch to New
Evidence-Based Guideline-Directed Therapy
for HFrEF …………………………… 205
2. How to Achieve Optimal Therapy Given Multiple
Drugs for HF Including Augmented Clinical
Assessment That May Trigger Additional Changes
in GDMT (e.g., Imaging Data, Biomarkers,
and Filling Pressures) ………………….. 211
3. When to Refer to an HF Specialist …………. 215
4. How to Address Challenges of Care Coordination . 215
5. How to Improve Adherence ……………… 216
6. What Is Needed in Specific Patient Cohorts:
African Americans, the Frail, and Older Adults … 218
7. How to Manage Your Patients’ Cost of Care for HF 219
8. How to Manage the Increasing Complexity of HF . 220
9. How to Manage Common Comorbidities …….. 222
10. How to Integrate Palliative Care and Transition to
Hospice Care ………………………… 2221. How to initiate, add, or switch therapy to new evidence-based guideline-directed treatments for HFrEF.
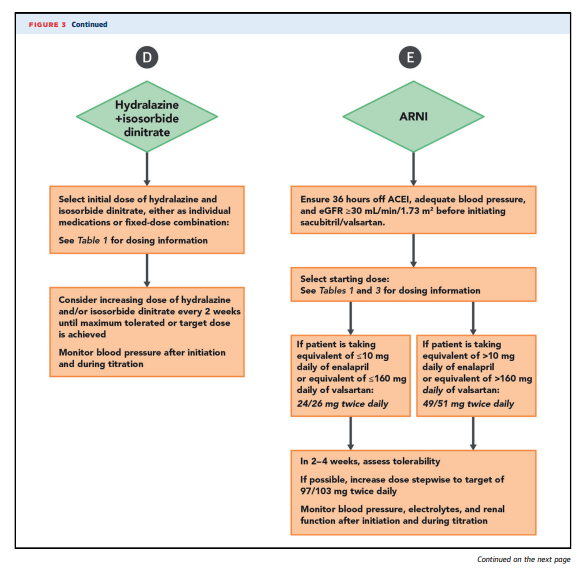
ARNI – angiotensin receptor – neprilysin inhibitor: Sacubitril/Valsartan from StatPearls:
Sacubitril/valsartan is the first agent to be approved in a new class of drugs called angiotensin receptor neprilysin inhibitor (ARNI). The medication is FDA-approved for the treatment of patients with chronic heart failure with reduced ejection fraction (HFrEF) with NYHA class II, III, or IV. Sacubitril/valsartan is to be used in place of an ACEI or angiotensin II receptor blocker (ARB) and in conjunction with other standard, heart-failure treatments (beta blocker, aldosterone antagonist).[1][2][3]
According to the 2016 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America (ACC/AHA/HFSA) Focused Update on New Pharmacological Therapy for Heart Failure, ACEI, ARB, or ARNI are now recommended in patients with chronic symptomatic HFrEF to reduce morbidity and mortality (class I recommendation).
Patients must be able to tolerate ACEI or ARB prior to being started on sacubitril/valsartan.[4]
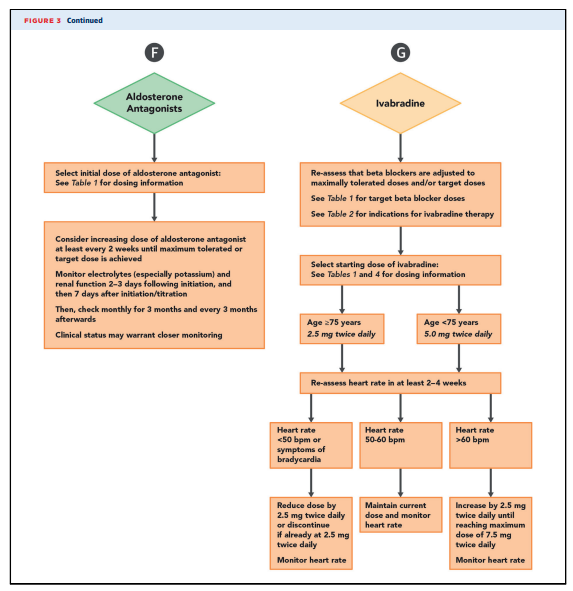
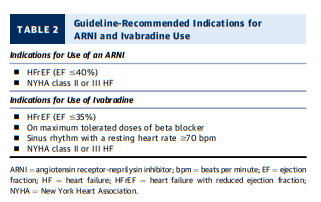
Ivabradine
Heart rate independently predicts outcomes in HFrEF. Evidence from beta-blocker trials suggests that heart rate lowering is directly related to improved outcomes (10). A dose-response relationship for evidence-based beta blockers used in HFrEF has been demonstrated (i.e., the higher the dose, the better the outcome). Prior to initiating any other agent with heart-rate slowing effects, the dose of an evidence-based beta blocker should be optimized. However, some apparently well-compensated patients on optimal beta blocker therapy continue to have a persistent resting heart rate over 70 bpm.
Ivabradine is an adjunctive means to reduce heart rate in
patients with chronic HFrEF who are in sinus rhythm. Ivabradine is a specific inhibitor of the If current involved in sinoatrial nodal activity and reduces the heart rate of patients in normal sinus rhythm without lowering blood pressure. In the SHIFT (Systolic HF Treatment with the If Inhibitor Ivabradine Trial) trial of 6,505 subjects with stable, chronic, predominantly NYHA class II and III HFrEF, ivabradine therapy, when added to GDMT, resulted in a significant reduction in HF hospitalizations (11). Benefits were noted especially for those patients with: contraindications to beta blockers, beta blocker doses <50% of GDMT targets (12), and resting heart rate $77 bpm at study entry (13). It is important to emphasize that ivabradine is indicated only for patients in sinus rhythm, not in those with atrial fibrillation, patients who are 100% atrially paced, or unstable patients. From a safety standpoint, patients treated with ivabradine had more bradycardia and developed more atrial fibrillation as well as transient blurring of vision (11).In the 2016 ACC/AHA/HFSA HF guidelines focused
update (3), ivabradine was recommended as a Class IIa,
Level of Evidence: B-R (1,2) therapy to reduce the risk of
HF hospitalization in patients with HFrEF (LVEF #35%)
already receiving GDMT (including a beta blocker at
maximally tolerated dose), and who are in sinus rhythm
with a heart rate greater than 70 bpm at rest (Figures 2 and 3,
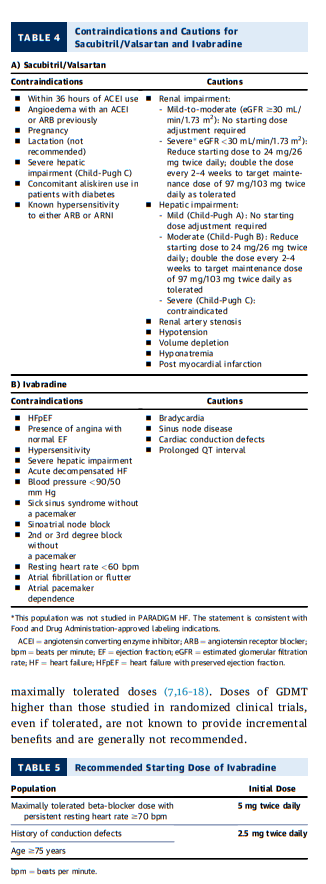
Tables 1 and 5). The contraindications to ivabradine are
enumerated in Table 4.Consensus Pathway Algorithm for Initiation and
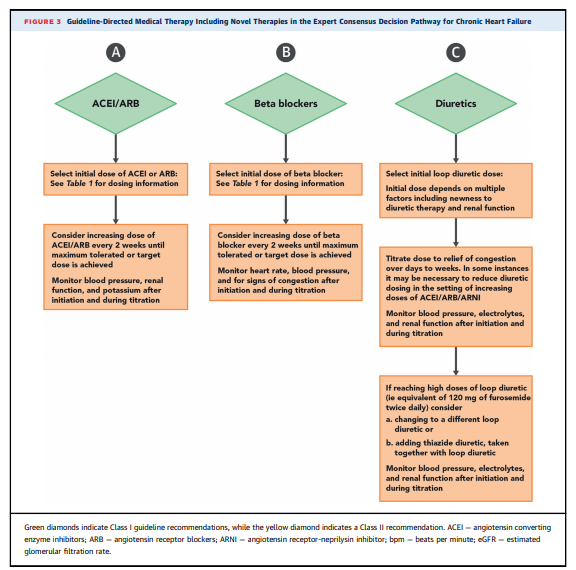
Titration of HFrEF TherapiesA strategy for initiating and titrating evidence-based
therapies for patients with HFrEF is depicted in Figures 2
and 3. As noted in the previous text, after a diagnosis of
HF is made, GDMT should be initiated and therapies should
be adjusted no more frequently than every 2 weeks to
target doses (or maximally tolerated doses). Clinicians
should aim to achieve this within 3 to 6 months of an initial
diagnosis of HF (however, this rapid timeline may not be
logistically feasible for some patients). GDMT should
continue to be up-titrated to achieve maximally tolerated
doses of these therapies. During follow-up, frequent reassessment of the clinical status of the patient, blood pressure, and kidney function (and electrolytes) should be performed. Reassessment of ventricular function should occur after target or maximally tolerated doses of GDMT are achieved for 3 months to determine the need for device
therapies such as implantable defibrillators and cardiac
resynchronization therapy (2). Structured medication titration plans embedded in disease management programs have been shown to be useful in obtaining target doses of GDMT within 6 months of hospital discharge (14)2. How to Achieve Optimal Therapy Given Multiple Drugs for
HF Including Augmented Clinical Assessment That May
Trigger Additional Changes in GDMT (e.g., Imaging Data,
Biomarkers, and Filling Pressures)Target Doses
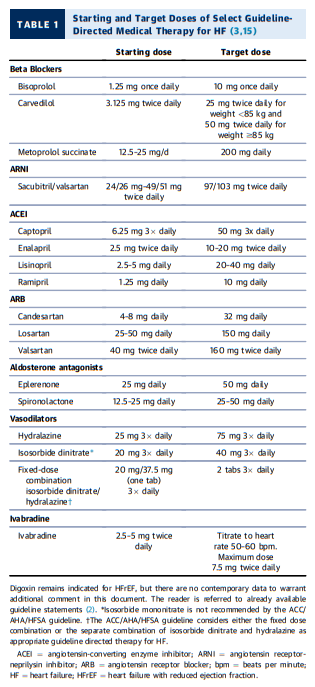
To achieve the maximal benefits of GDMT in patients with
chronic HFrEF, therapies must be initiated and titrated to maximally tolerated doses (7,16–18). Doses of GDMT
higher than those studied in randomized clinical trials,
even if tolerated, are not known to provide incremental
benefits and are generally not recommended.Strategies for titration are detailed in Figures 2 and 3. [Above] Achieving target or maximally tolerated doses of GDMT is
the goal.Beta-blocker doses should be adjusted every 2 weeks (19) in a patient with no evidence of decompensated HF and no contraindications to higher doses.
ACEI and ARB may be titrated similarly to beta blockers with monitoring of renal function, potassium, and blood pressure; more rapid titration is also reasonable in clinically stable patients.
In the absence of hypotension, electrolyte/renal instability, or angioedema on an ACEI or ARB, it is reasonable to change to ARNI.
For those taking ARNIs, doses can be increased every 2 to 4 weeks to allow time for adjustment to the vasodilatory effects of the combined angiotensin receptor and neprilysin inhibition while also monitoring renal function, potassium, and especially blood pressure.
For optimal titration of ACEI, ARBs, or ARNI, lower loop
diuretic doses may be necessary to permit titration; in
this circumstance, careful attention to potassium concentrations is needed, as the kaliuretic effects of loop
diuretics may no longer be present, and restriction of
supplemental and/or dietary potassium may be
necessary.Aldosterone antagonists are added in patients with chronic HFrEF already receiving beta blockers and ACEI/ARB/ARNI who do not have contraindications to this therapy (2). It is not necessary to achieve target or maximally tolerated doses of other drugs before adding aldosterone antagonists. The dose of aldosterone antagonists used in clinical trials, which is typically
below that which might influence blood pressure, is sufficient for clinical efficacy. Adherence to the guideline recommendations for monitoring of renal function and potassium is required.For a number of reasons, HYD/ISDN-indicated therapy for HF is often neglected in eligible patients. However, given the benefits of this combination (43% relative reduction in mortality and 33% relative reduction in HF hospitalization [20]), African-American patients should receive these drugs once target or
maximally tolerated doses of beta blocker and ACEI/ ARB/ARNI are achieved (2). This is especially important for those patients with NYHA class III to IV symptoms.Finally, following assiduous titration of beta blockers, in patients whose heart rate remains > 70 bpm on target or maximally tolerated doses of beta blockers, ivabradine (3) can be added and titrated every 2 weeks to lower heart rate.
Barriers to Medication Titration
In some instances, it may not be possible to titrate GDMT
to the target doses achieved in clinical trials.Abnormal renal function and/or hyperkalemia are
common barriers to initiation and titration of GDMT.For patients with established renal disease, caution may
be necessary when starting GDMT, though ACEI/ARB are
generally considered safe in patients with creatinine
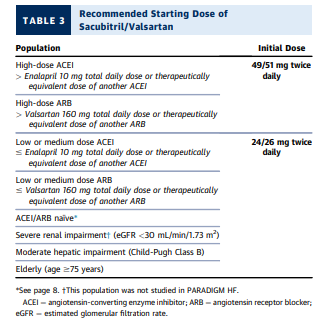
<3.0 mg/dL.In patients with mild-moderate renal impairment (eGFR > 30 mL/min/1.73 m2 and <60 mL/min/1.73 m2), no adjustment is needed when deciding the starting dose of the ARNI sacubitril/valsartan.
In those with severe renal impairment (eGFR <30 mL/min/1.73 m2), the starting dose of sacubitril/valsartan should be reduced
to 24/26 mg twice daily (This population was not studied in
PARADIGM HF. The statement is consistent with FDA approved labeling indications) (Table 4). [Above]Aldosterone antagonists are contraindicated in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2, or creatinine >2.5 mg/dL in men or creatinine >2 mg/dL in women) or with potassium >5.0 mEq/dL (Figure 1).
Renal function and potassium should be assessed within 1 to 2 weeks of the initiation or dose increase of ACEI/ARB/ARNI.
In patients with preserved renal function or mild to moderate renal impairment, renal function and potassium after initiation and titration of aldosterone antagonists should be assessed within 2 to 3 days and again at 7 days. The schedule for subsequent monitoring should be dictated by the clinical stability of renal function and volume status but should occur at least monthly for the first 3 months and every 3 months thereafter (2).
After the initiation or titration of loop diuretics, renal function should be assessed within 2 to 3 days.
During initiation and titration of agents that affect renal function, a decrease in eGFR of >30% or the development of hyperkalemia should alert the clinician that a reduction in doses may be necessary, even though short-term changes in eGFR during intense diuretic therapy or with the initiation of ACEI or ARB do not predict longer-term adverse outcomes (21).
In patients with evidence of hypovolemia, the dose of diuretics
should be reduced.Doses of ARNI may also need to be reduced in the setting of renal insufficiency or hypotension.
Hyperkalemia may also require changes in medical therapy.
Clinical assessment and renal stability in each patient dictates whether clinicians may need to monitor certain patients more closely than others.
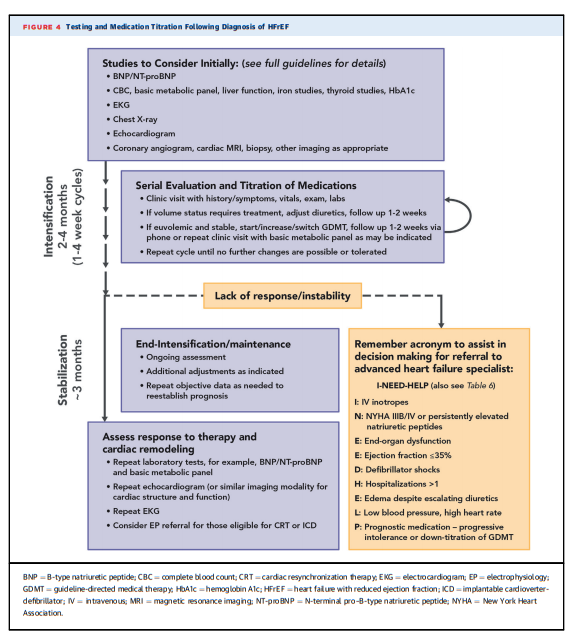
Clinical Assessment
Figure 4 details a reasonable strategy for patient evaluation and management following a diagnosis of HFrEF.
After GDMT is initiated and titrated with the goal of achieving clinical trial doses or maximally tolerated doses, patients with chronic HFrEF should be evaluated on a regularly scheduled basis. For most patients, areasonable interval is every 3 to 6 months, although many may require more frequent follow-up to monitor clinical stability and revisit opportunities for further GDMT titration.
Cardiac rehabilitation is beneficial and remains underutilized.
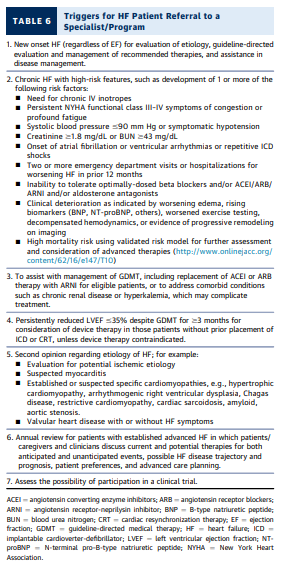
High-risk features (conveniently summarized in the
acronym “I NEED HELP” in Figure 4 and Table 6) should
trigger consideration for referral for advanced HF consultation (22); features triggering referral to advanced HF care are also discussed in the answers to Issue 3 and Table 6.
Biomarkers—When to Order Natriuretic Peptides
B-type natriuretic peptide (BNP) and N-terminal pro–B type natriuretic peptide (NT-proBNP) are the most studied biomarkers in HF. They play a role in diagnosis and
prognostication. Higher concentrations of BNP or NTproBNP in an ambulatory patient with HFrEF informs
high risk, particularly when the concentrations are rising.Current clinical practice guidelines give a Class I recommendation to measure NT-proBNP or BNP to support a clinical diagnosis of HF, to assess disease severity, or to establish prognosis (2).
More recently, biomarkers have been examined for
their role as a marker of clinical responsiveness to GDMT.
This is, in part, due to the fact that a wide range of GDMT
may reduce BNP and NT-proBNP concentrations, in parallel with the benefits of these therapies. Patients whose
natriuretic peptide concentrations do not fall with GDMT
(“nonresponders”) have a worse prognosis and more
deleterious LV remodeling (23). Therefore, measurement
of BNP or NT-proBNP is useful to monitor risk, to assist in
decision making regarding the ordering of imaging
studies to evaluate LV remodeling, to support clinical judgment with respect to prescription of GDMT, and to provide helpful objective data regarding decision-making for referral to advanced HF therapies (See Figure 4 and Table 6). Concentrations of BNP or NT-proBNP are supported with a Class I guideline recommendation to determine prognosis. In the setting of worsening symptoms (24), the reassessment of BNP or NT-proBNP may be informative. However, serial assessment of BNP or NTproBNP to guide aggressive titration of GDMT is not
indicated and not warranted (25). Severe renal dysfunction may interfere with the interpretation of natriuretic peptide concentrations.While rising natriuretic peptide concentrations are
correlated with adverse outcomes, this relationship can
be confounded with the use of sacubitril/valsartan. Due to
neprilysin inhibition, concentrations of BNP rise in patients treated with sacubitril/valsartan and tend not to return to baseline despite chronic therapy. In contrast, NT-proBNP concentrations typically decrease, as NTproBNP is not a substrate for neprilysin (26). Therefore, clinicians should interpret natriuretic peptides in the context of GDMT; BNP concentrations will increase (while NT-proBNP will most often fall) with ARNI therapy, and thus it may be more prudent to check only NT-proBNP in patients on ARNI. Also, transient increases in natriuretic peptide levels have been documented in the initial phases of beta-blocker initiation; such changes should not preclude up-titration of beta-blocker therapy, which should
be guided by patient tolerance instead of asymptomatic change in natriuretic peptide levels.Filling Pressure Assessment—When and How to Measure Filling Pressures
Whereas routine pulmonary artery catheterization is not
recommended to manage congestion, invasive hemodynamic and filling pressure assessment may occasionally be useful to support decision making.
However, there are now also POCUS techniques to determine right sided filling pressures. See Dr. Rola’s 2019 book, Bedside Ultrasound: a primer for clinical integration, 2nd Edition.
And also see Link To Dr. Rola’s “POCUS Assessment Of Venous Congestion” With A Demo Of The VExUS Exam
Posted on November 2, 2020 by Tom Wade MD