Important Note: As I occasionally remind myself and my readers, this website and the posts are my study notes on medicine (mainly), business topics, and other subjects. I find that creating the posts helps me reinforce my learning.
The complete 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure [Link to PDF] needs to be reviewed in its entirety. This article contains information on heart failure that must be known by outpatient clinicians as well as hospital-based clinicians.
In this post I link to and excerpt from:
2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure: A Report of the American College of Cardiology Solution Set Oversight Committee. [PubMed Abstract] [Full Text HTML] [Full Text PDF]
Hollenberg SM, Warner Stevenson L, Ahmad T, Amin VJ, Bozkurt B, Butler J, Davis LL, Drazner MH, Kirkpatrick JN, Peterson PN, Reed BN, Roy CL, Storrow AB.J Am Coll Cardiol. 2019 Oct 15;74(15):1966-2011. doi: 10.1016/j.jacc.2019.08.001. Epub 2019 Sep 13.PMID: 31526538 No abstract available.
And here are excerpts:
3.1. Definition
GDMT: Guideline-directed medical therapy
Optimal therapy: Treatment provided at either the
target or the highest tolerated dose for a given patient.
EF: Ejection fraction
HFrEF: Heart failure with reduced left ventricular
ejection fraction (EF ≤0.40)
HFpEF: Heart failure with preserved left ventricular
ejection fraction (EF ≥0.50)
HFmrEF: Heart failure with midrange ejection fraction
(EF <0.50 but >0.40)
Text
Text
Text
6. NODE: ADMISSION
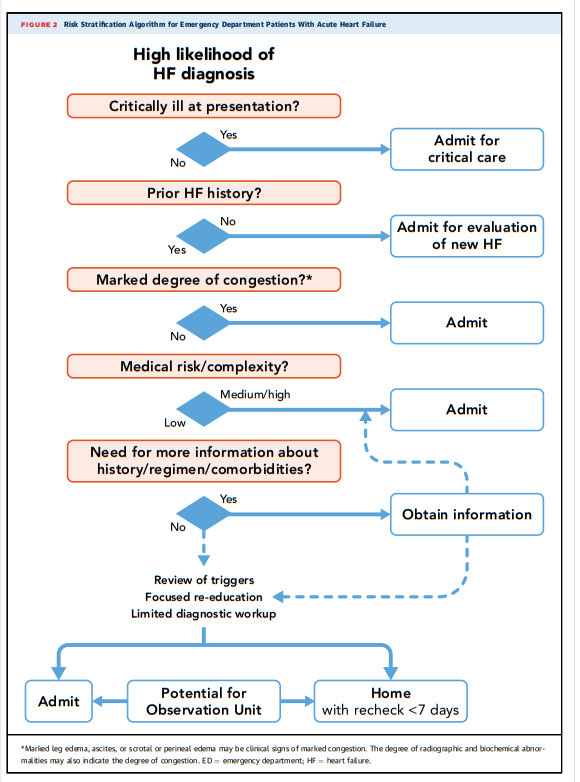
6.1. Evaluation in the ED
[Review Table 1 above] ED data show that 80% of all HF hospitalizations are admitted from the ED (27,28). Although many advances have improved chronic HF management, there is sparse evidence regarding strategies for triage and management in the ED (13,15–19,25,26,29,30). Most patients with acute decompensated heart failure (ADHF) are admitted for symptomatic treatment of congestion with intravenous
diuretics and to a much lesser degree for respiratory
failure, cardiogenic shock, incessant ventricular tachycardia, or the need for urgent diagnostic or therapeutic procedures (6,20,21,31–40). Although fewer than 10% of
ED visits with ADHF have acute life-threatening illness,
and the majority of patients presenting are clinically stable (11,38,39,41), the post-discharge event rate is high
even though over 80% to 90% of patients are admitted
(28,42,43).Early therapy for acute HF is crucial even if patients are
ultimately admitted. Medical therapy is discussed in
Sections 7.2, 7.4, 7.5, and 7.6. Diuretic dosing for
decongestion is considered in detail in Section 7.2.6.2. Comprehensive Initial Assessment—
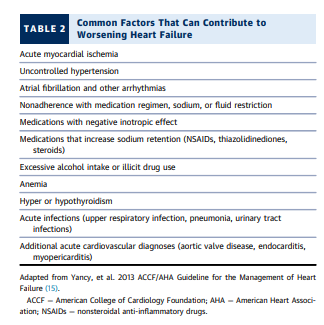
Setting the Inpatient GoalsThe two central themes of care for patients hospitalized
for decompensated HF are decongestion and optimization
of the therapies recommended for HF, but multiple other
goals also need to be met. The coordinated care plan includes evaluation as necessary of the primary etiology of
the heart disease and potential aggravating factors that
would require specific intervention, both cardiac and
noncardiac (34) (see Table 2 [above]).6.2.1. Assessing Hemodynamic Profiles
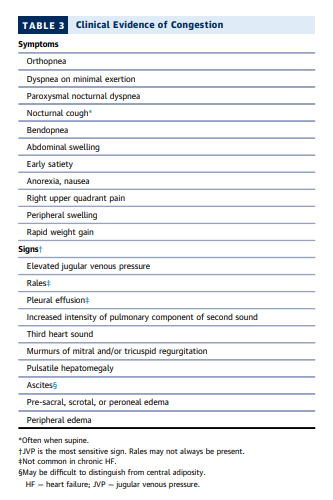
Most patients present with at least 1 symptom and 1 sign
of congestion that can be tracked as targets during
decongestion and may serve as sentinel symptoms for
recurrent congestion after discharge (70,74,77,78)
(Table 3). The jugular venous pressure (JVP) reflects
elevated right-sided filling pressures and is also a sensitive indicator of elevated left-sided filling pressures in
patients with HF (75,79). Rales, when present, usually
indicate higher filling pressures than baseline, but are
often absent in chronic HF due to pulmonary lymphatic
compensation. Extensive pitting edema, ascites, or large
pleural effusions reflect large extravascular reservoirs
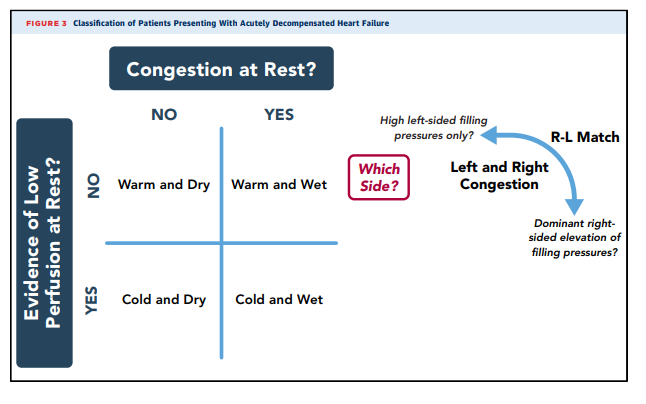
that may take many days to mobilizeClinical profiles of patients with HF are shown in Figure 3.
Patients identified with congestion should be further considered for whether filling pressures are elevated in proportion for
both the right heart and the left heart (right atrial pressure >10
mm Hg and pulmonary capillary wedge pressure >22 mm Hg;
75% to 80% of patients with chronic HFrEF, less defined for
HFpEF) (42,80). The wet and warm clinical profile without
evidence of hypoperfusion characterizes over 80% of patients
admitted with reduced EF and almost all with preserved EF
except those with small left ventricular cavities of restrictive or
hypertrophic cardiomyopathies (39,42).The cold and wet profile describes congestion accompanied by clinical evidence of hypoperfusion, as suspected from narrow pulse pressure, cool extremities, oliguria, reduced alertness, and often recent intolerance to neurohormonal inhibition. Sleepiness, impaired concentration, and very low urine output may also be present. These patients may require adjunctive therapy with vasodilator or inotropic agents or decrease of medications with negative inotropic effects to improve cardiac output and facilitate diuresis.
Patients who appear to have low cardiac output
without clinical congestion (cold and dry profile) often have
unrecognized elevation of filling pressures, which may be
revealed by invasive hemodynamic measurement. Uncertainty
regarding hemodynamic status is associated with worse outcomes and is an indication for invasive hemodynamic assessment (15,81). True hypoperfusion without elevated filling
pressures accounts for fewer than 5% of admitted patients (39)
and usually reflects aggressive prior therapy with tight adherence. A patient hospitalized with apparent decompensation in whom both filling pressures and perfusion appear to be normal should be carefully evaluated for other causes of symptoms, such as transient ischemia or arrhythmias, or noncardiac diagnoses such as pulmonary disease.
Please note that the images in this post are PNGs. The reader should go to Table 4 in the article PDF for direct active links to the Guidelines/Pathways referenced in the image below.
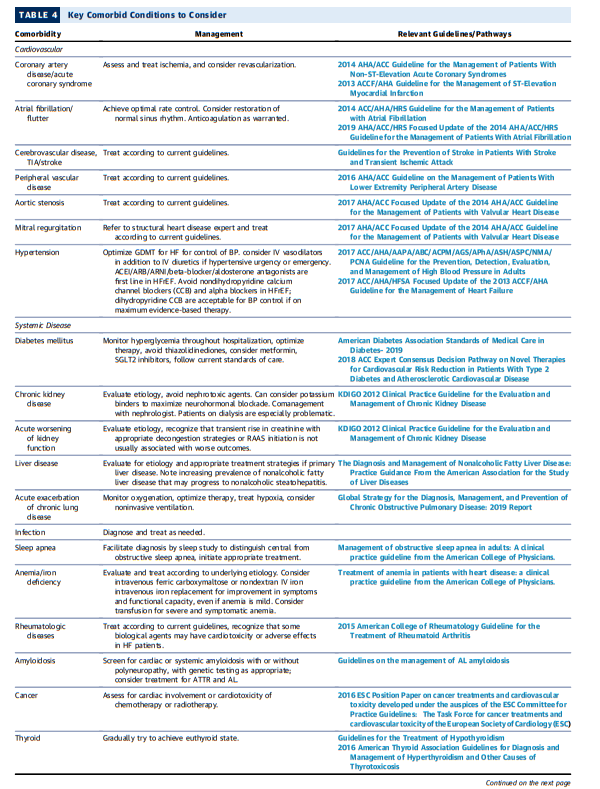
6.2.2. Consideration of Comorbidities
A key component of the comprehensive initial assessment is
evaluation of patient comorbidities (Table 4).These comorbidities and their therapies should be carefully considered for their role in HF decompensation and as independent targets for intervention. For example, diabetes mellitus and pulmonary disease are each present in 30% to 40% of patients hospitalized with HF and play a role in disease severity and risk for decompensation (82).
Kidney dysfunction can precipitate congestion and can also limit initiation of GDMT.
Frailty* is another common comorbidity in HF, particularly for
the elderly (83,84), and its association with health, functional
status, and late-life disability is an increasingly important
focus for patients with HF and their caregivers. Approximately
50% to 70% of older patients admitted with ADHF present
with some degree of frailty, although this may be reversed or
attenuated with interventions (85,86). Consideration shoul be given at the time of hospitalization to the need for physical
therapy consultation.*See Clinical Frailty Scale in an Acute Medicine Unit: a Simple Tool That Predicts Length of Stay [PubMed] [Full Text HTML] [Full Text PDF]. Can Geriatr J. 2016 Jun; 19(2): 34–39.
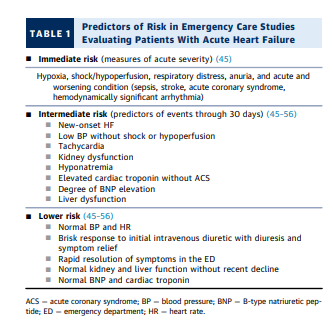
6.2.3. Initial Risk Assessment
[Note to myself: This section is so full of clinical pearls that it just needs to be reviewed in its entirety.]
[Really, the whole document should be reviewed completely from time to time because it is full of information that is relevant to outpatient clinicians caring for heart failure patients.]
Because a key message of this document is the importance
of serial assessment from admission through discharge, the
risk factors listed in Table 5 are categorized according to the
time when they may be known during the hospitalization. In
setting goals to decrease risk and improve outcomes after
hospitalization and later, it may be helpful to focus on those
risk factors most likely to be modifiable.At any time between admission and discharge, recognition of high risk for unfavorable outcomes should trigger
specific considerations (Table 6), including caution
regarding the initiation of therapies that may be difficult
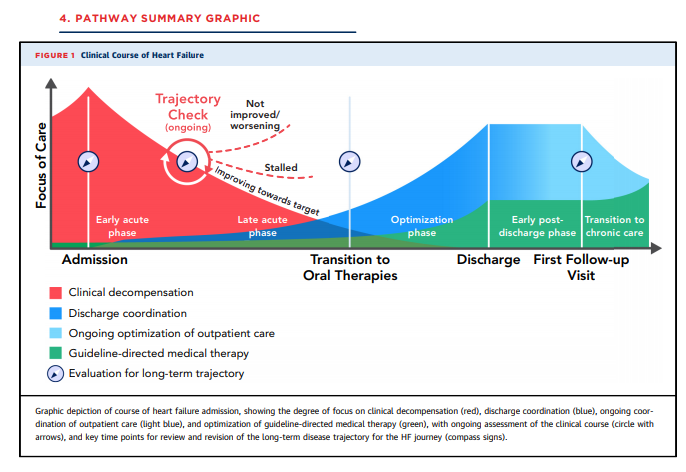
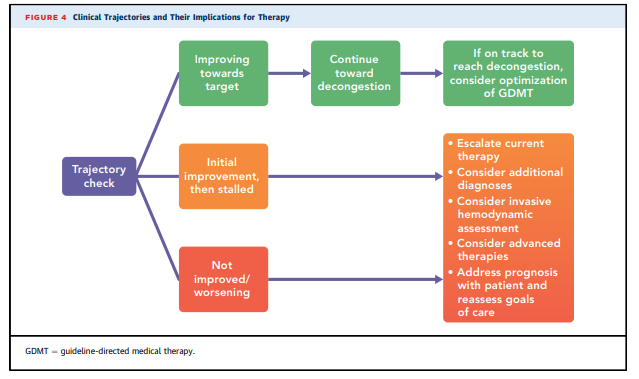
to discontinue.7. NODE: DAILY TRAJECTORY CHECK
Three main in-hospital trajectories have been defined
according to changes in patient symptoms, clinical signs,
laboratory markers and imaging if done, presence or
absence of complications, assessment and treatment of
comorbidities, and treatment alignment with goals
of care: 1) improving towards target; 2) stalled after
initial response; or 3) not improved/worsening. These trajectories translate into different management strategies
throughout the hospitalization and post-discharge
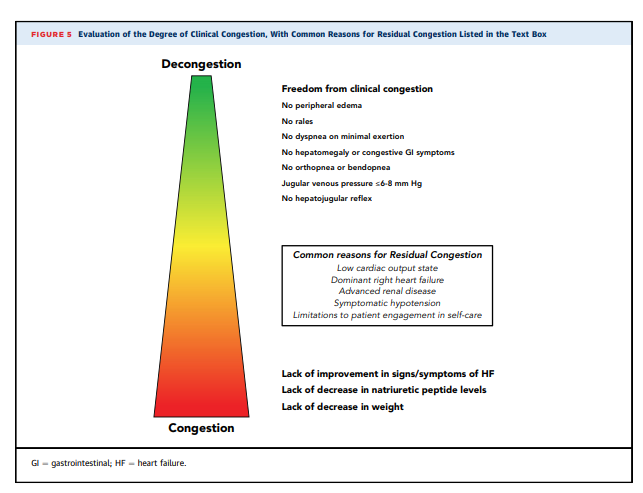
(Figure 4).7.1. Targets for Decongestion
Inpatient trajectories are primarily defined by the pace and
extent of decongestion. Evaluation of the degree of clinical
congestion is depicted in Figure 5. The usual goal is
for complete decongestion, with absence of signs and
clinical symptoms of elevated resting filling pressures
(70,78,117,130). Rates of rehospitalization and death are
consistently lower in patients rendered free of clinical
congestion by the time of discharge (67,78). National
Heart, Lung, and Blood Institute–sponsored trials of ADHF
have specified goals of resolution of edema, orthopnea,
and jugular venous distention (74,77,78,131,132).JVP should generally be reduced to <8 cm, dyspnea at rest
should be relieved, and there should be no residual orthopnea, bendopnea, or edema (77,78). Peripheral reservoirs of anasarca, large pleural effusions, and ascites as detectable should gradually be depleted, after which intravascular filling pressures as indicated by JVP will more rapidly decrease. The amount of net diuresis that will be needed for complete decongestion cannot be ascertained at the time of admission, and the difference between admission weight and a previous target weight often underestimates the excess fluid. Postural hypotension is often interpreted as indication of overdiuresis,but frequently reflects overvasodilation.Most patients report early improvement in symptoms,
particularly dyspnea. In admissions with HFrEF, shortness of breath was reported as the worst symptom by about one-half of patients, fatigue by about one-third, and abdominal discomfort, swelling, or edema by the remaining patients.The magnitude of patient-reported improvement was least for patients with a worst symptom of fatigue.
Average weight loss in recent inpatient HF trials ranges from 4 to 8 kg (74,77).
Substantial reduction in B-type natriuretic peptide levels is
anticipated during effective diuresis, frequently decreasing by
50% or more from admission (111), and a decrease in natriuretic
peptide concentrations of at least 30% before discharge is
strongly associated with better outcomes.Kidney function is not a reliable biomarker for volume status or
change in volume status; modest increases are not linked to
worse outcomes as long as the rise in creatinine is transient
(143,144) and accompanied with successful decongestion (118-
120,123), or occurs after initiation of renin-angiotensin system
(RAS) or aldosterone antagonists (145–147).The targets for decongestion may need modification for
mismatch of right and left and right-sided filling pressures
(Figure 3, right side).Approximately 70% to 75% of patients with decompensated chronic HFrEF have concordance of relative right and left filling pressures around thresholds of right atrial pressure of 10 mm Hg and pulmonary capillary wedge pressure of 22 mm Hg (75,80).
Clinical assessment can be helpful to confirm or challenge concordance (42), but clinical evidence for elevated left-sided pressures may be subtle in the presence of prominent right-sided findings. Information from recent invasive studies or echocardiographic hemodynamic evaluation should be brought forward to inform the hemodynamic targets. Patients in whom elevated right atrial pressures approach or exceed left-sided filling pressures often cannot undergo diuresis to a normal JVP and may be more likely to receive inotropic support (79). Conversely, patients with elevated left-heart pressures in the presence of normal right-sided pressures may continue to have
orthopnea and dyspnea on minimal exertion despite diuresis to JVP in the normal range; their optimal rightsided pressures may be in the lower range of normal.7.2. Diuretic and Adjunctive Therapy [Carefully review this entire section in the article for details on the use of diuretic therapy]
Establishing an effective diuretic regimen is crucial for
achieving decongestion. Usually patients require the first
dose of intravenous (IV) diuretics at presentation or in the
ED, and IV diuretics are continued throughout the hospitalization until effective decongestion warrants transition
to oral diuretics before discharge.During hospitalization, electrolytes should be measured at least daily and corrected. Similarly, daily weights, patient intake and urine output, kidney function by measurement of serum creatinine, and BUN should be monitored. Serum creatinine commonly rises slightly during effective decongestion (143,144), but generally returns to baseline early after discharge, and is not associated with worse outcomes (118–120,123).
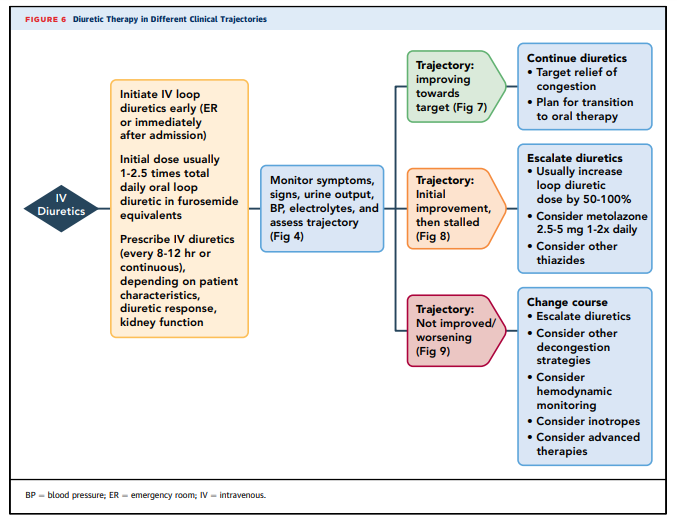
7.3. TRAJECTORY: Improving Towards Target
Net fluid loss and weight loss are expected with intravenous diuretics, usually at least 1 kilogram of weight loss per day (77,78,137,139).
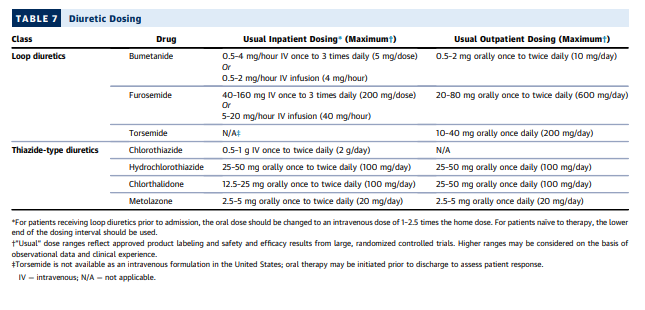
Diuretic doses should be titrated as necessary (Figure 6, Table 7).
When sufficient progress has occurred to render it
likely that targets of decongestion will be reached, it is usually appropriate to initiate or up-titrate components of the GDMT regimen (see Sections 7.4 and 8.3).7.4. Optimization of GDMT
Neurohormonal antagonists have dramatically improved
outcomes for HFrEF. When possible, continuation of
GDMT through hospitalization or initiation before discharge is associated with substantially better outcomes, both due to the benefit of the therapies and to the better prognostic profile of patients who can tolerate them (106).Expert advice concerning initiation of GDMT for chronic HF can be found in the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart
Failure Treatment (13).**2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to
10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction. A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways [PubMed Abstract]* [Full Text HTML] [Full Text PDF]. JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY VOL. 71, NO. 2, 2018.*To reinforce my learning of the above article I made excerpts of the article in my post This Pathway Is For Outpatient Heart Failure: Links To And Excerpts From “2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment.
Continuing 7.4 Optimization of GDMT
Hospitalization provides a pivotal opportunity to decrease risk and improve clinical trajectory in patients who respond well
to diuresis and who have not previously received adequate trials of GDMT. This therapy modifies and frequently reverses disease progression (9). This therapy modifies and frequently reverses disease progression (9).The introduction of GDMT during hospitalization for HF with
reduced ejection fraction is thus a key target to reduce risk (9,160). This has been shown for ACEI, beta blockers, and most recently supported for angiotensin receptor– neprilysin inhibitor (ARNI).Patients with good early response to diuresis should be considered for addition of recommended therapies or up-titration toward trial targets for neurohormonal antagonist therapy as decongestion is approached, recognizing that the diuretic response may diminish acutely with increasing neurohormonal antagonism, particularly if blood pressure is lowered.
The 2017 ACCF/AHA guidelines emphasize that “caution
should be used when initiating beta blockers in patients
who have required inotropes during their hospital course
or when initiating ACEIs, ARBs or aldosterone antagonists
in those patients who have experienced marked azotemia
or are at risk for hyperkalemia” (26).For these reasons [above], expectations regarding the prescription and dosing of GDMT in patients with ADHF are more conservative and individualized than for stable patients in outpatient HF management.
For patients with HFpEF, beyond diuretics, clinical trial evidence that medical therapy improves outcomes is limited, but it seems reasonable to titrate RAS inhibitors to desired blood pressures in hospital.
One important common principle is to start at a
low dose and titrate slowly upward as tolerated (Table 1 in
Yancy et al. [13]). High starting doses and/or overly
aggressive titration can result in hypotension and worsening kidney function, setbacks that limit both decongestion and initiation of different components of GDMT.7.4.1 RAS Therapy
RAS inhibition is part of GDMT for patients with HFrEF, and should be continued or initiated in the absence of hypotension or unstable kidney function. If prior therapy was held during hospitalization, lower doses may be required when therapy is resumed.
RAS inhibition can decrease blood pressure in patients with pre-existing intense neurohormonal activation, so particular care should be taken in patients recently weaned from intra-venous inotropic therapy or in those diuresed extensively prior to its initiation. Caution should be exerted also inpatients with acute kidney injury or hyperkalemia(143).
Discharge information to the outpatient clinician should include a reminder to considermreinitiation of neurohormonal therapies stopped in the hospital.
7.4.2. Angiotensin Receptor–Neprilysin Inhibitors
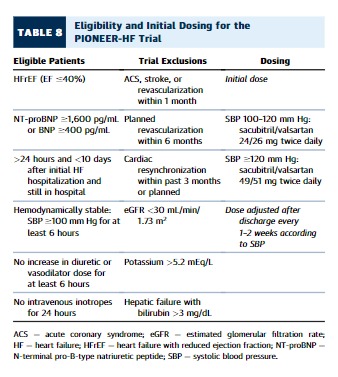
Current recommendations include ACEI, ARB, and ARNIas approved inhibitors of the RAS in chronic HF(164).Although there is extensive experience with initiation of ACEI and ARB as recommended therapies for hospitalized HF, the pivotal PARADIGM-HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial for ARNIfocused exclusively on stable chronic HF, and excluded patients recovering from acute decompensated HF; thistrial also included a run-in period with enalapril(165). The PIONEER-HF (Comparison of Sacubitril-Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode) trial now provides evidence to support safety of careful initiation of sacubitril-valsartan for hospitalized patients with and without prior exposure to ACEI or ARB, selected for hemodynamic stability, with systolic blood pressure > 100mm Hg and without escalation of intravenous diuretics or vasodilators for 6 hours, and without intravenous inotropic therapy within the previous 24 hours.(166).Compared withpatients started on enalapril, patients randomized to sacubitril/valsartan 24/26 mg twice daily(or 49/51 mg for SBP > 120 mm Hg) had more reduction in N-terminal pro–B-type natriuretic peptide (BNP) levels and in the exploratory clinical outcome of HF rehospitalization, similar to previous results in the outpatient setting(167).
7.4.3 Beta Blockers
In patients with HF with the wet and warm profile who are taking beta blockers on admission, they should generally be continued unless blood pressure is low. If HF remains re-fractory to diuretics, the dose should be halved. Discontinuation should be considered if congestion remains unresponsive and certainly if the addition of intravenous inotropic therapy is contemplated.
If decreased or held, beta blockers may be initiated or resumed in the absence of symptomatic hypotension or bradycardia, but a margin of stability is required in view of the known acute effects to lower cardiac output and increase filling pressures.
Low doses and slow up-titration are recommended for a patient after recent decompensation.
In the case of metoprolol, it is reasonable to give test doses of 6.25 mg of the short-acting metoprolol tartrate, but escalation of short-acting doses maybe paradoxically less tolerable due to higher and more rapid peak effects(168).
Alternatively, test doses of carvedilol 3.125 mg may be administered. Planned testing of increased doses of beta blockers should be part of the treatment plan either during the index hospitalization or following discharge.
In hospitalized patients in whom GDMT medications have been held or not initiated, the optimal sequence of reinitiation of ACEI and beta blockers has not been established, although outpatient studies suggest that that either an ACEI or betablocker may be initiated first(169,170).
Consideration should be given to initiating or resuming beta blockers after decongestion, particularly in patients with more advanced disease or those in whom other GDMT has been titrated.
Patients who have required temporary IV inotropic therapy during hospitalization represent a higher-risk cohort and require longer periods of observation prior to and after beta blocker initiation.When it has been difficult to wean inotropic therapy, use of beta blockers is often deferred until stability has been confirmed after discharge.
7.4.4. Aldosterone Antagonists
Start here.