The posts in this blog are mostly my study notes on medical education or business topics I am currently studying. And I use my version of Spaced Repetition* to fix the topic in memory.
*Spaced repetition: a hack to make your brain store information, January 23, 2016 from The Guardian.
First, I review the relevant article, chapter, or blog post. Then I’ll listen to the accompanying podcast. And finally I’ll excerpt parts of the article by pasting portions in my blog. This method helps me fix the concepts in my mind.
In this post I link to and excerpt from the chapter, Fluid selection & pH-guided fluid resuscitation, November 2, 2016, of Dr. Farkas’ incredible Internet Book Of Critical Care [Link is to TOC]. And after reviewing the chapter, listen to the 33 minute summary podcast of the chapter.
For this post, I first watched Dr. Farkas’ YouTube video (12 min) pH-guided fluid resuscitation [Link is to the YouTube video]. Then I reviewed Dr. Farkas IBCC chapter titled Fluid selection & pH-guided fluid resuscitation. Then I listened to Dr. Farkas’ summary podcast of the chapter.
And finally I excerpted the chapter.
Dr. Farkas has created this Table of Contents of the chapter and each heading is a direct link to that part of the chapter:
CONTENTS
All that follows are excerpts from the pH-guided fluid resuscitation Chapter of The Internet Book Of Critical Care [except where noted – material in brackets]:
forward
Several years ago, the ICU at Genius General Hospital transitioned from using normal saline to using mostly Lactated Ringers (spoiler alert: it was neither difficult nor dramatic). More recently, the use of pH-guided resuscitation has become increasingly common.
Fluid choice probably doesn’t make much difference for most patients. However, fluid therapy is an extremely common intervention. When leveraged over the high number of patients receiving fluid, even small differences in efficacy can be important (e.g. NNT of 30 or 50). Finally, for occasional patients with significant pre-existing hyperkalemia or metabolic acidosis, fluid choice may be extremely important.
crystalloids versus colloids
role of colloids (albumin)?
Currently, albumin seems to be indicated primarily for the purpose of supporting renal function among patients with cirrhosis, including:
- Management of spontaneous bacterial peritonitis.
- Management of hepatorenal syndrome.
- Prophylaxis against hepatorenal syndrome after large volume paracentesis.
hetastarch is poison [NEVER use it]
- There is no medicolegal or evidence-based justification for using hetastarch.
step I: balanced crystalloid
rationale for transitioning from normal saline to balanced crystalloids
- (1) There was never any physiologic rationale to use normal saline in the first place. Most reasons offered to support the use of saline aren’t based on physiology or evidence (e.g. “it’s cheap” or “it’s what we’re used to using”).
- (2) Normal saline exacerbates acidosis. This may be problematic – especially in patients who are severely acidotic to begin with (which isn’t uncommon among critically ill patients).
- (3) Normal saline has been shown to exacerbate hyperkalemia (by causing acidosis which shifts potassium out of cells) (O’Malley 2005, Khajavi 2008, Modi 2012, Weinberg 2017).
- (4) In animal models, normal saline causes significant harm compared to balanced crystalloid (e.g. greater acidosis, impaired cardiac function, coagulopathy, impaired renal function, and mortality)(Kellum 2004, Orbegozo 2016 ). In humans, two RCTs have shown that saline may cause hemodynamic instability, compared to balanced crystalloids (Potura 2015, Pfortmueller 2018).
- (5) Hyperchloremia caused by normal saline may cause renal vasoconstriction, increasing the risk of kidney injury. This has been shown in a variety of studies, most recently the SALT-ED RCT.
- Interestingly, the SALT-ED trial showed benefit from balanced crystalloid, despite most patients’ receiving relatively little fluid.
arguments for using saline & why they lack merit [See the chapter for why.]
ongoing studies on saline versus balanced crystalloids
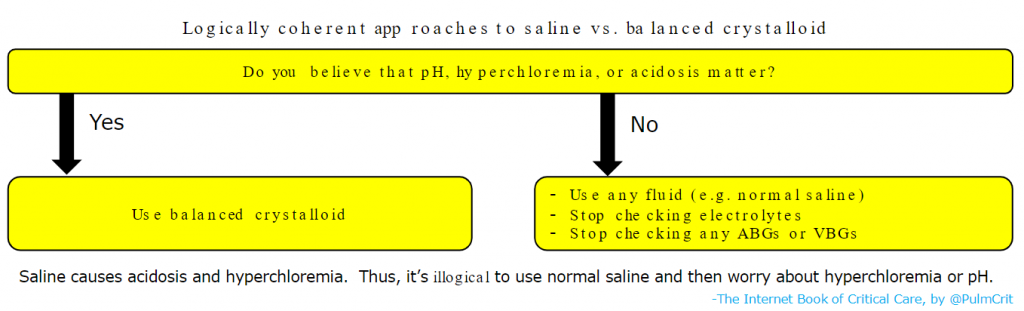
- Further studies are ongoing regarding the selection of saline versus balanced crystalloids. However, it’s dubious whether we really need any additional trials (Vincent 2016):
- There is zero physiologic rationale for using saline in most patients.
- Nearly all available physiologic, animal, and clinical data suggests balanced crystalloids are superior.
- It’s well established that normal saline will cause acidosis and hyperchloremia (this is a fact).
- At this point, there are only two logically coherent strategies which exist, as shown below:
choice of balanced crystalloid
discussion of various anions
- Sodium lactate
- Historically, administration of lactate was feared (due to worsening of “lactic acidosis”). This isn’t possible, because sodium lactate isn’t an acid.
- Lactate may function as a metabolic fuel for the heart, so if anything, lactate could be a good thing. Hypertonic sodium lactate infusion has been shown to improve cardiac function (Nalos 2014).
- In vivo, lactate will be very rapidly metabolized into bicarbonate by the liver (unless the patient has fulminant hepatic failure).
- Sodium acetate
- Rapidly metabolized into bicarbonate.
- Lacks lactate’s beneficial cardiac effects. Excessive acetate levels may cause vasodilation and hypotension, but this doesn’t seem to be clinically relevant (acetate will be rapidly metabolized and only transiently present).
- Sodium gluconate
- Although often believed to be metabolized into bicarbonate, this doesn’t seem to be the case – so sodium gluconate does not function as an alkali (unlike sodium acetate and sodium lactate). This means that Plasmalyte has the same exact pH effect as Lactated Ringers.
- Sodium gluconate appears to be cleared unchanged from the kidneys. It could even function as an osmotic diuretic agent.
contraindications to Lactated Ringers
- These are not legitimate contraindications:
- Hyperkalemia (more on this here).
- Cirrhosis or liver injury (unless the patient has frank hepatic failure, it will be able to metabolize lactate).
- Legitimate contraindications (all relative however):
- Elevated intracranial pressure – Lactated Ringers could theoretically worsen this, because it is slightly hypotonic. Giving a liter of lactated ringers will have roughly the same effect as giving a liter of normal saline plus a dose of medication mixed in ~150 ml D5W. So this isn’t a huge issue, but it’s not ideal either. For a patient with known elevation of intracranial pressure, plasmalyte would be preferable.
- Metformin-associated lactic acidosis – In this clinical scenario patients genuinely may have difficulty metabolizing the lactate. Note, however, that lactated ringers contains sodium lactate – so it will increase the lactate level without causing acidosis (more on this here).
- Severe hypercalcemia – Lactated Ringers has 1.5 mM of calcium, which won’t worsen hypercalcemia (if anything it could decrease the calcium level, because this will be a lower calcium concentration than the patient’s blood). However, this isn’t the optimal fluid here (more on this here).
- Overall, the contraindications to lactated ringers are generally uncommon and fairly mild. Outside of a neurological ICU, LR would be an excellent choice for ~95% of patients and a safe choice for nearly all patients.
choice of best balanced crystalloid?
- Differences between various balanced crystalloids are minor and probably of minimal clinical significance.
- Lactated Ringers is generally an outstanding choice as it is inexpensive, widely available, and physiologically sound (the choice of lactate as an anion is arguably superior to gluconate/acetate).
- Plasmalyte is also an excellent choice, which may be superior in situations where Lactated Ringers is relatively contraindicated (listed above).
step II: pH-guided resuscitation
general concept
- The transition from normal saline to balanced crystalloids (Step I, above) is focused largely on the avoidance of harm from fluid (e.g. hyperchloremia). However, we can take this concept a step further to use crystalloids to improve the pH status of selected patients.
- Fluid should be viewed as a drug.
- Just as we wouldn’t give the patient “any antibiotic” we shouldn’t give “any fluid” – the fluid should be selected to maximize benefit.
- Fluid resuscitation is a limited opportunity to manipulate pH status.
- Large volumes of fluid can be used to affect the patient’s pH status.
- After the patient is volume resuscitated, this opportunity will be lost (because large volumes of fluid can no longer be given without causing volume overload).
pH abnormalities treatable with crystalloid
- (1) Non-anion-gap metabolic acidosis (NAGMA)
- This essentially represents a bicarbonate deficit (whether bicarbonate has been lost in the stool or urine).
- Patients with normal kidneys will eventually re-generate bicarbonate, but this takes time. Furthermore, critically ill patients frequently have renal insufficiency or renal tubular acidosis, which prolong recovery from NAGMA.
- Exogenous bicarbonate administration is a physiologically logically and reasonably well-accepted treatment for NAGMA.
- (2) Uremic metabolic acidosis
- Most forms of anion-gap metabolic acidosis (e.g. lactic acidosis or ketoacidosis) don’t respond favorably to IV bicarbonate. One exception to this rubric may be uremic metabolic acidosis.
- Exogenous bicarbonate has long been used by nephrologists in efforts to improve pH and avoid dialysis. This practice was recently validated in the BICAR-ICU trial, wherein bicarbonate administration decreased the requirement for dialysis in uremic patients (more on this here).
- (3) Acute metabolic alkalosis
- Most forms of metabolic alkalosis seen in the ICU are chronic (e.g. chronic compensatory metabolic alkalosis in response to chronic respiratory acidosis). Compensatory alkalosis should be left alone.
- Very rarely, acute metabolic alkalosis may be seen. For example, this may be caused by ingestion of large quantities of alkali, large volume diuresis (contraction alkalosis), or gastric losses (vomiting, continuous NG suction).
- Normal saline is a rational therapy for acute metabolic alkalosis, because it will reduce the serum bicarbonate level back towards normal.
- Note that the following abnormalities are not treatable with crystalloid:
- Chronic metabolic alkalosis which is compensatory for a chronic respiratory acidosis.
- Anion-gap metabolic acidoses other than uremia (e.g. lactic acidosis or ketoacidosis).
pH-guided resuscitation
- This is pretty simple – it largely amounts to thinking about the patient’s pH status and whether choice of IV fluid could improve it.
- When giving bicarbonate, the bicarbonate deficit may be a useful guide:
- Bicarbonate deficit (in mEq) can be estimated this calculator from MDCalc.
- Each liter of isotonic bicarbonate contains 150 mEq of bicarbonate (more on this below).
- Generally, avoid giving the patient more than roughly ~80% of their bicarbonate deficit, to prevent over-correction of the metabolic acidosis.
- During a bicarbonate shortage, sodium acetate can be used in its place.
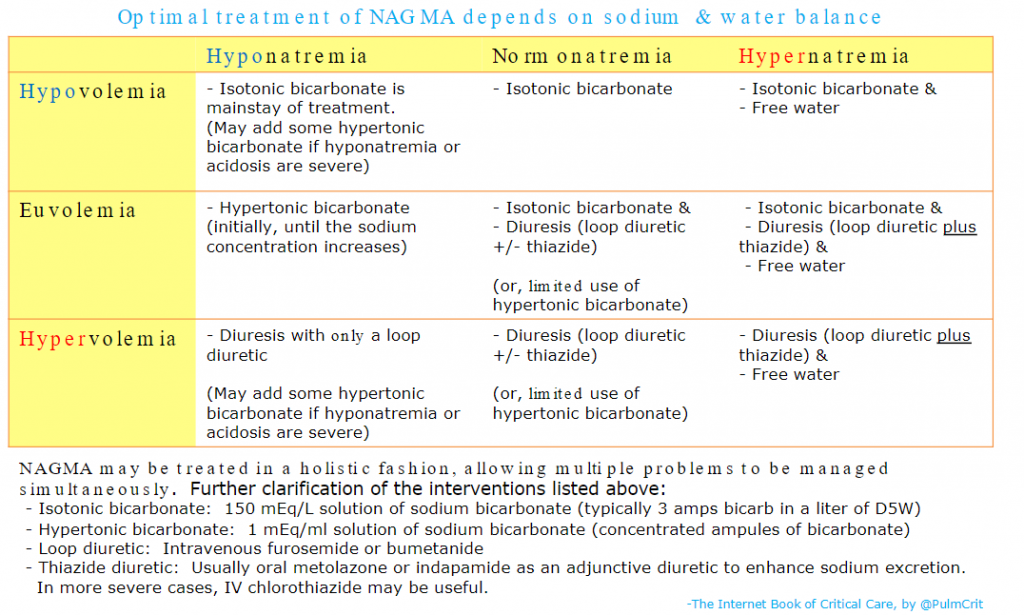
And here is the chart from the Treatment section of Dr. Farkas’ IBCC chapter, Non-Anion Gap Metabolic Acidosis with instructions on preparing and using Isotonic Bicarbonate:
Returning now to excerpts from the IBCC chapter Fluid selection & pH-guided fluid resuscitation:
pH-guided resuscitation is most important in uremic metabolic acidosis
- This is probably the most common situation where pH-guided resuscitation is beneficial.
- Isotonic bicarbonate may improve the pH and help avoid dialysis. Alternatively, if the patient is resuscitated to a euvolemic state without isotonic bicarbonate, it will become impossible to provide them with an adequate amount of bicarbonate (these patients are often oliguric, so further fluid could provoke pulmonary edema).
- These patients are often hyperkalemic – a process which will also be alleviated by isotonic bicarbonate (discussed further in the chapter on hyperkalemia).
- Overall, there is a subset of patients with acute kidney injury, uremic metabolic acidosis, and hyperkalemia who will respond very favorably to isotonic bicarbonate with resolution of their electrolytic problems. This may buy them some time for their kidneys to recover, potentially avoiding the need for dialysis.
hypertonic & isotonic bicarbonate
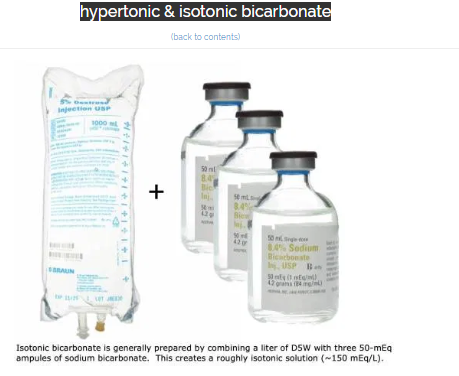
what is isotonic bicarbonate?
- Isotonic bicarbonate is generally formulated by adding 150 mEq of sodium bicarbonate to a liter of D5W (above).
- Although the bag of fluid will be hypertonic, glucose doesn’t function as an effective osmole (since it readily enters cells). Therefore, in vivo this solution will behave as an isotonic fluid.
- D5W is used as the base solution because most hospitals don’t have IV sterile water available. If your hospital does have IV sterile water, this would be preferable to D5W to produce a pure isotonic solution of bicarbonate.
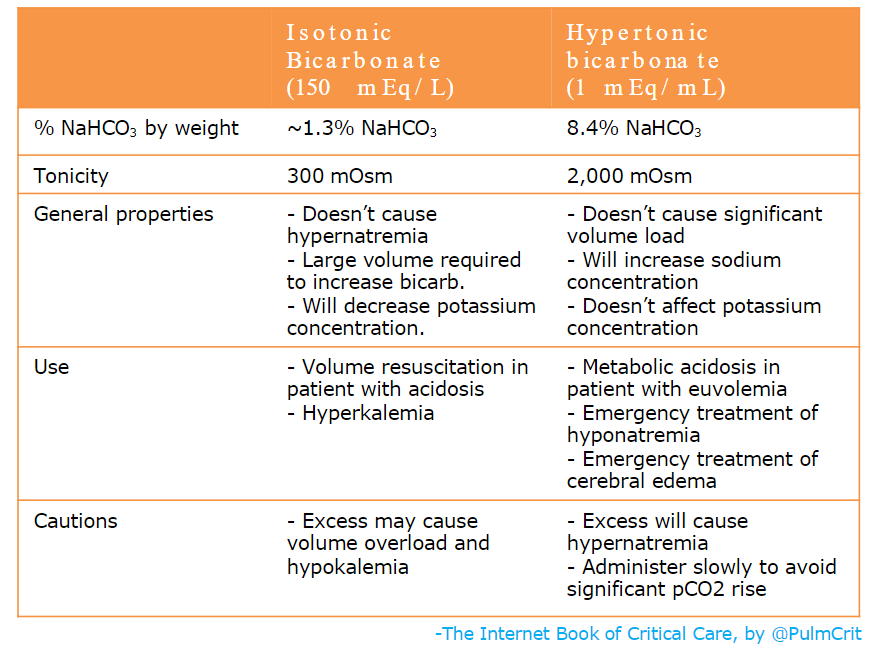
isotonic bicarbonate vs. hypertonic bicarbonate
- The most commonly used forms of bicarbonate are hypertonic bicarbonate (undiluted ampules) and isotonic bicarbonate, as compared above.
- The amount of hypertonic bicarbonate which can be given is limited by the sodium concentration. Each 50-ml ampule of bicarbonate will increase the sodium concentration by roughly ~1-1.5 mEq/L. Caution needs to be excercised with repeated ampules, as eventually this may cause hypernatremia.
- The amount of isotonic bicarbonate which can be given is generally limited by volume overload. Each 150 mEq of bicarbonate comes along with a liter of volume.
dissolved CO2 & how rapidly can isotonic bicarbonate be given?
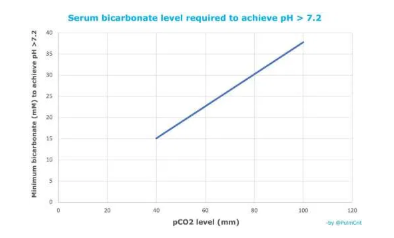
- Intravenous bicarbonate contains both bicarbonate and dissolved CO2. For example, the concentration of pCO2 in an ampule of bicarbonate may be ~100 mm.
- Following administration:
- Dissolved CO2 will transiently increase the patient’s pCO2. Over time, this will be breathed off and the patient will return to their prior pCO2 level. This will happen even if the patient is on mechanical ventilation (administered pCO2 increases the gradient driving CO2 out of the body – which increases CO2 clearance and eventually returns the patient to their baseline pCO2). The only situation where CO2 doesn’t return to baseline is if the patient has died (e.g. cardiac arrest, with minimal effective circulation).
- Bicarbonate will persist longer, after the pCO2 has been exhaled. This explains the alkalinizing effect of IV bicarbonate.
- Fun fact: the pH of an ampule of bicarbonate is only 8. It’s not that alkaline in the bottle (which contains both pCO2 and bicarbonate). The reason it causes alkalinization in vivo is because pCO2 is breathed off while bicarbonate remains.
- Ampules of sodium bicarbonate generally shouldn’t be pushed over a few seconds. This may cause rapid pH shifts, including elevated pCO2. Thus, ampules of hypertonic bicarbonate should generally be pushed slowly over ~5-10 minutes if possible.
- Isotonic bicarbonate can be infused at rates similar to other crystalloids (e.g. 75-1,000 ml/hr). Given the lower concentration of CO2 in isotonic bicarbonate, rapidly loading the patient with CO2 isn’t an issue here.
effect on potassium concentration
- Three factors are in play here:
- (1) Hypertonicity causes potassium to shift out of cells (a process known as solute drag).
- (2) Bicarbonate increases the pH, which shifts potassium into cells.
- (3) Volume load of isotonic bicarbonate may directly dilute out potassium, thereby decreasing the potassium concentration.
- Hypertonic bicarbonate
- Factors #1 & #2 cancel each other out.
- Several RCTs have shown that hypertonic bicarbonate does not affect potassium level.
- Isotonic bicarbonate
- Factors #2 & #3 both serve to reduce the potassium level.
- Available data shows that isotonic bicarbonate decreases the potassium level among patients with metabolic acidosis (Blumberg 1992, Fraley 1977, Gutierrez 1991).
- Clinical significance depends on what you’re trying to achieve:
- Hyperkalemia: If you’re trying to reduce the potassium level, you need to use isotonic bicarbonate.
- Hypokalemia: If you’re trying to increase the pH without dropping the potassium, then hypertonic bicarbonate could have an advantage here. Alternatively you could use isotonic bicarbonate with simultaneous potassium supplementation.
hypocalcemia
- Increases in pH will tend to decrease the ionized calcium level (essentially removal of protons stuck to albumin renders albumin more negatively charged, leading to an increase in calcium-albumin binding).
- Increasing the pH to a normal range shouldn’t cause hypocalcemia, but it may exacerbate pre-existing hypocalcemia.
common errors with bicarbonate [And How To Avoid Them]
- In general, it’s desirable to standardize this fluid in order to avoid medication errors:
- (a) Don’t mix up a solution with two ampules of bicarbonate. If you want to give the patient some additional D5W, it’s preferable to run two simultaneous infusions (one with D5W and another with true isotonic bicarbonate).
- (b) Don’t mix up 3 ampules of bicarbonate in a liter of normal saline!
- Don’t be afraid to run isotonic bicarbonate at the rate you need. For example, in a severely hypovolemic patient who needs fluid and bicarbonate, you may wish to run the isotonic bicarbonate at 250-1,000 ml/hr (to provide both volume and bicarbonate).
- Don’t slam in an ampule of hypertonic bicarbonate (unless there is a really good reason, such as profound tricyclic intoxication).
- Don’t use hypertonic bicarbonate to treat hyperkalemia (proven not to work).
- Don’t bolus hypertonic bicarbonate for a patient in cardiac arrest (unless you suspect a toxicologic etiology).
- Don’t use bicarbonate to treat lactic acidosis or ketoacidosis (this doesn’t work and gives bicarbonate a bad reputation).
therapeutic alkalization to augment permissive hypercapnia
basic concept
- Occasionally, intubated patients who are encountered who are extremely difficult to ventilate (typically due to status asthmaticus or severe ARDS).
- The safest approach to these patients may be to administer exogenous bicarbonate, with a goal of increasing the bicarbonate level to ~30-35 mEq/L
- Note that the normal level of bicarbonate in blood is 22-28 mEq/L. Thus, a serum bicarbonate level of 30-35 mEq/L isn’t terribly high.
- This will generally amount to shifting patients from a state of mild metabolic acidosis (most patients start off with a bicarbonate of ~20 mEq/L) to mild metabolic alkalosis. Higher serum bicarbonate makes it easier to safely ventilate patients (targeting a pH >7.15-7.20). Bicarbonate administration may be safer than increasing the respiratory rate or tidal volume (maneuvers which will increase mechanical force delivered to the lungs and may also increase the risk of pneumothorax).
- Note that the development of a pneumothorax in a patient with profound ARDS or asthma may be a catastrophic event.
- Left to their own devices, patients with ARDS or status asthmaticus will often eventually compensate for their respiratory acidosis by mounting a compensatory metabolic alkalosis. Exogenous bicarbonate administration aims to achieve the same thing, merely accelerating this normal adaptation process.
- There is no high-quality evidence on this topic. The use of exogenous bicarbonate to balance out severe respiratory acidosis is a longstanding practice in critical care (e.g. utilized in the classic ARMA trial on ARDS). Unfortunately there is no clear data to guide the speed and magnitude of alkalization which may be optimal.
how to achieve therapeutic alkalization
- Depending on the patient’s weight and baseline bicarbonate, this will generally involve administration of ~150-300 mEq sodium bicarbonate to target a serum bicarbonate level of ~30-35 mEq/L. This should generally be achieved gradually over a period of several hours.
- Hypertonic bicarbonate: Some or all of this exogenous bicarbonate may be administered in the form of hypertonic sodium bicarbonate (8.4%, described above). Hypertonic bicarbonate has the advantage of limiting added volume, but it will eventually cause hypernatremia.
- Isotonic bicarbonate: This may be useful in patients with hypovolemia or hypernatremia. In a patient with euvolemia and high-normal sodium, isotonic bicarbonate could be combined with diuretics (e.g. furosemide and thiazide diuretics) to achieve alkalinization without causing volume overload.
- A thiazide diuretic may be useful here to promote sodium excretion and avoid hypernatremia (more on this here). Furosemide alone tends to cause excretion of dilute urine – so the combination of furosemide plus isotonic bicarbonate may still tend to increase the patient’s sodium level.
- The optimal rate of alkalinization is unknown, and likely varies depending on the individual patient scenario. In most cases, gradual alkalization (e.g. 25-100 mEq bicarbonate per hour) is sufficient.
- Bicarbonate administration will cause a transient increase in pCO2 during its administration, which will cause a transient reduction in pH. However, once completed, pCO2 will decrease to baseline and the added bicarbonate will increase the pH.
- If bicarbonate is administered more slowly, then transient pCO2 elevations are smaller. Of course, it will take longer to get to target pH.
- This issue of dissolved CO2 is discussed further in the above section in IV bicarbonate.
Pitfalls
- Don’t use normal saline as your default resuscitative fluid. There are many reasons for this, but one salient one is as follows: eventually you will wind up giving liters of saline to a hyperkalemic and acidotic patient, thereby pushing them off a pH cliff.
- Don’t be afraid to use Lactated Ringers in patients with hyperkalemia or liver dysfunction. Don’t be afraid to use Plasmalyte in any patient (there don’t seem to be any legitimate contraindications to Plasmalyte).
- Don’t miss opportunities to fix your patient’s pH abnormalities using pH-guided resuscitation (especially for patients with uremic metabolic acidosis).
- Not understanding how to use various forms of bicarbonate.