In this post I link to and excerpt from Episode 176.0 – Pneumonia Updates, Jan 27, 2020. Hosts: Brian Gilberti, MD and Audrey Tse, MD, from Core EM.
In their podcast, Drs Gilberti and Tse discuss Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
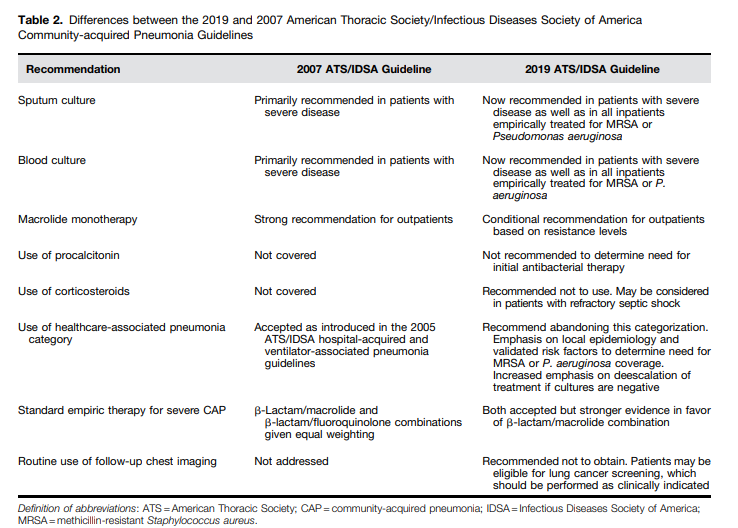
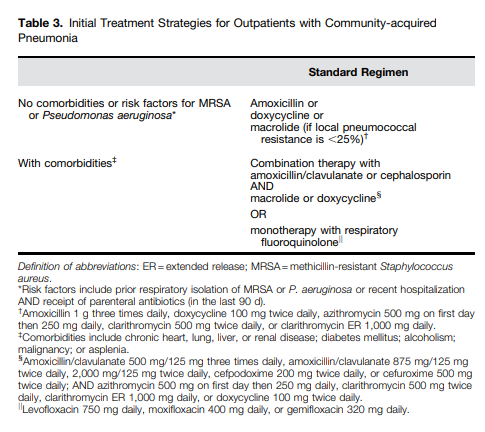
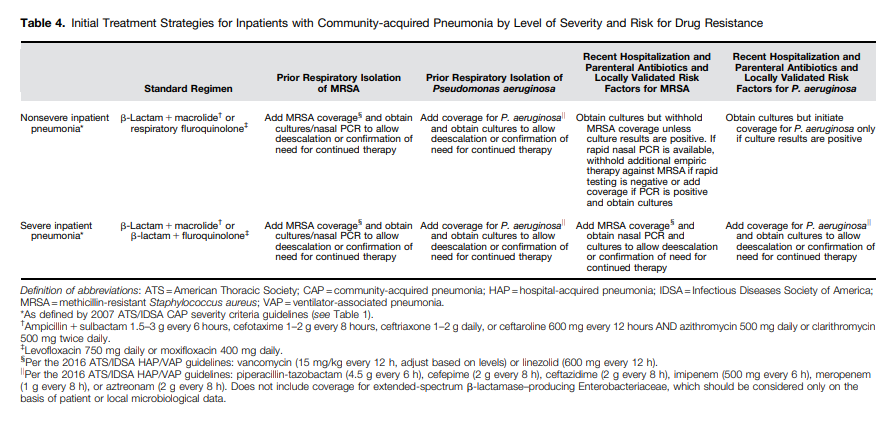
I posted on the above Clinical Practice Guideline in Links To And Summary Of The 2019 Guidelines For Community Acquired Pneumonia With Additional Resources. Posted on January 5, 2020 by Tom Wade MD.
If you want a quick summary of the Clinical Practice Guideline go to my post above.
And so in this post I excerpt from Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67:
Question 3: In Adults with CAP, Should Legionella and Pneumococcal Urinary Antigen Testing Be Performed at the Time of Diagnosis?
Recommendation. We suggest not routinely testing urine for pneumococcal antigen in adults with CAP (conditional
recommendation, low quality of evidence), except in adults with severe CAP (conditional recommendation, low quality of evidence).We suggest not routinely testing urine for Legionella antigen in adults with CAP (conditional recommendation, low quality
of evidence), except1. in cases where indicated by epidemiological factors, such as association with a Legionella outbreak or recent travel (conditional recommendation, low quality of evidence); or
2. in adults with severe CAP (see Table 1) (conditional recommendation, low quality of evidence).
We suggest testing for Legionella urinary antigen and collecting lower respiratory tract secretions for Legionella culture on selective media or Legionella nucleic acid amplification testing in adults with severe CAP (conditional recommendation, low quality of evidence).
Question 4: In Adults with CAP, Should a Respiratory Sample Be Tested for Influenza Virus at the Time of Diagnosis?
Recommendation. When influenza viruses are circulating in the community, we recommend testing for influenza with a rapid influenza molecular assay (i.e., influenza nucleic acid amplification test), which is preferred over a rapid influenza diagnostic test (i.e., antigen test) (strong recommendation, moderate quality of evidence).
Rationale for the recommendation. The benefits of antiviral therapy support testing of patients during periods of high influenza activity. During periods of low influenza activity, testing can be considered but may not be routinely performed. Of note, this testing recommendation has both therapeutic and infection-control implications in the hospital setting. Updated
influenza testing recommendations are also available on the CDC website (https://www.cdc.gov/flu/professionals/diagnosis/index.htm) .Question 7: Should a Clinical Prediction Rule for Prognosis plus Clinical Judgment versus Clinical
Judgment Alone Be Used to Determine Inpatient General Medical versus Higher Levels of Inpatient
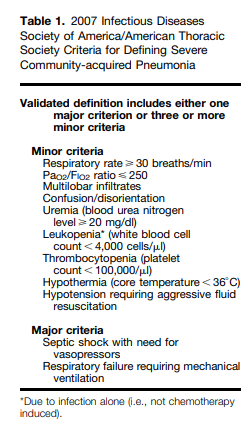
Treatment Intensity (ICU, StepDown, or Telemetry Unit) for Adults with CAP?Recommendation. We recommend direct admission to an ICU for patients with hypotension requiring vasopressors or
respiratory failure requiring mechanical ventilation (strong recommendation, low quality of evidence).For patients not requiring vasopressors or mechanical ventilator support, we suggest using the IDSA/ATS 2007 minor severity criteria (Table 1) together with clinical judgment to guide the need for higher levels of treatment intensity (conditional recommendation, low quality of evidence).
Rationale for the recommendation. Patients transferred to an ICU after admission to a hospital ward experience
higher mortality than those directly admitted to the ICU from an emergency department (64–67). This higher mortality
may in part be attributable to progressive pneumonia, but “mis-triage” of patients with unrecognized severe pneumonia may
be a contributing factor (64).It seems unlikely that physician judgment alone
would be equivalent to physician judgment together with a severity tool to guide the site-of-care decision. We recommend the 2007 IDSA/ATS severe CAP criteria* over other published scores, because they are composed of readily available severity
parameters and are more accurate than the other scores described above.*See Table 1 above.
Question 10: In the Inpatient Setting, Should Patients with Suspected Aspiration Pneumonia Receive
Additional Anaerobic Coverage beyond Standard Empiric Treatment for CAP?Recommendation. We suggest not routinely adding anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected (conditional recommendation, very low quality of evidence).
Question 13: In Adults with CAP Who Test Positive for Influenza, Should the Treatment Regimen Include Antiviral Therapy?
Recommendation. We recommend that antiinfluenza treatment, such as oseltamivir, be prescribed for adults with CAP who test positive for influenza in the inpatient setting, independent of duration of illness before diagnosis (strong recommendation, moderate quality of evidence).
We suggest that antiinfluenza treatment be prescribed for adults with CAP who test positive for influenza in the outpatient setting, independent of duration of illness before diagnosis (conditional recommendation, low quality of evidence).
Question 14: In Adults with CAP Who Test Positive for Influenza, Should the Treatment Regimen Include Antibacterial Therapy?
Recommendation. We recommend that standard antibacterial treatment be initially prescribed for adults with clinical and radiographic evidence of CAP who test positive for influenza in the inpatient and outpatient settings (strong recommendation, low quality of evidence).