In addition to the article referenced below, please see #159 Atrial Fibrillation Review and Update [The link is to the podcast and show notes], JULY 8, 2019 By CYRUS ASKIN from The Curbsiders.
What follows are links and excerpts from 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Am Coll Cardiol. 2019 Jul 9;74(1):104-132. doi: 10.1016/j.jacc.2019.01.011. Epub 2019 Jan 28.
Here are some excerpts:
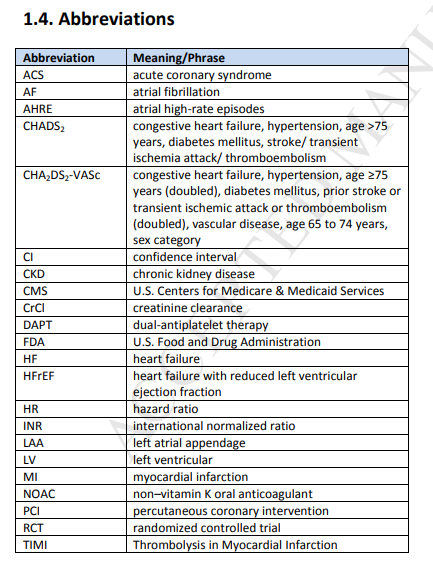
Class of Recommendation and Level of Evidence
The Class of Recommendation (COR) indicates the strength of recommendation, encompassing the estimated magnitude and certainty of benefit in proportion to risk. The Level of Evidence (LOE) rates the quality of scientific evidence supporting the intervention on the basis of the type, quantity, and consistency of data from clinical trials and other sources (Table 1) (P-5).
1. Introduction
The purpose of this document is to update the “2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation” (S1.3-1) (2014 AF Guideline) in areas for which new evidence has emerged since its publication.
All recommendations (new, modified, and unchanged) for each updated clinical section are included to provide a comprehensive assessment. The text explains new and modified recommendations, whereas recommendations from the previous guideline that have been deleted or superseded no longer appear. Please consult the full-text version of the 2014 AF Guideline (S1.3-1) for text and evidence tables supporting the unchanged recommendations and for clinical areas not
addressed in this focused update.
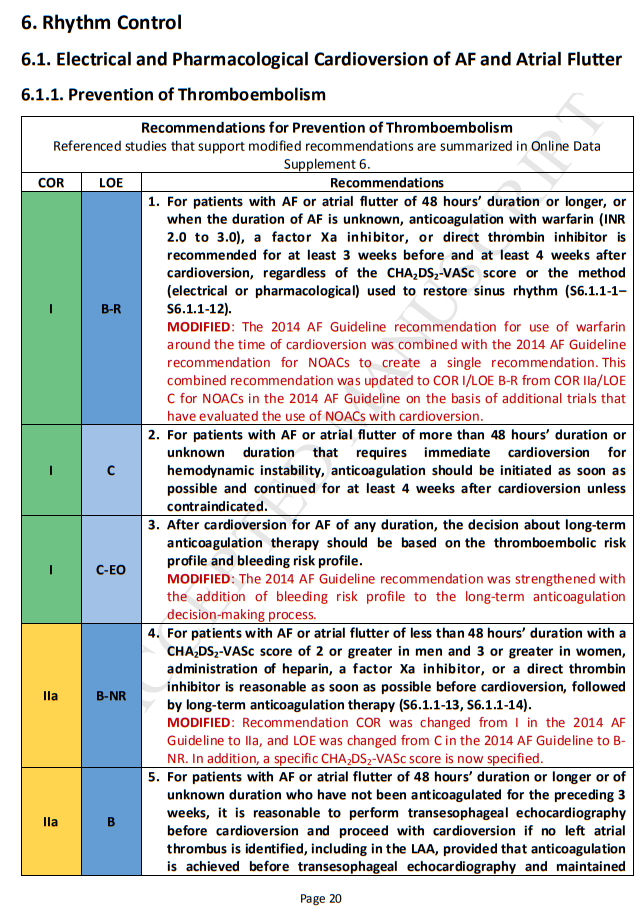
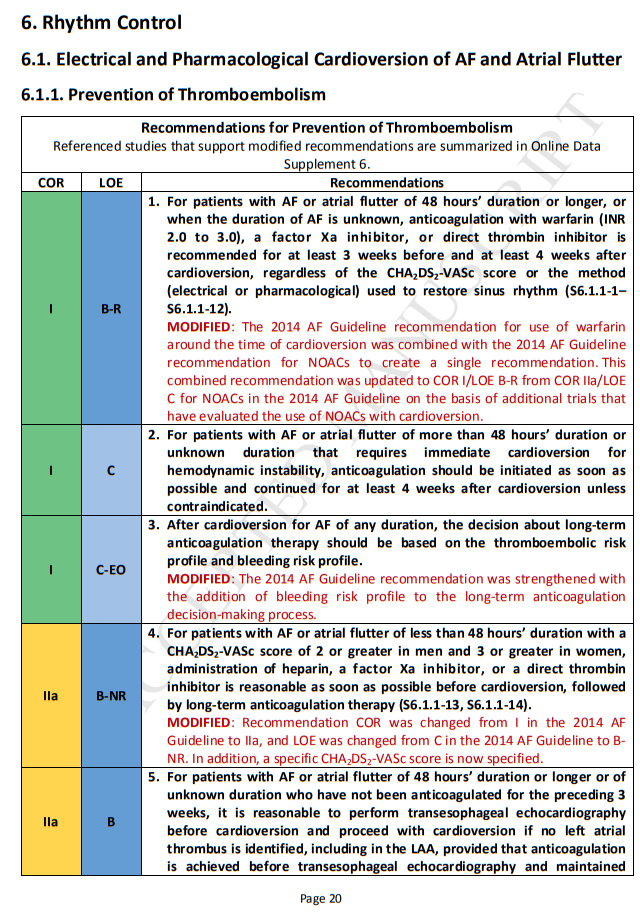
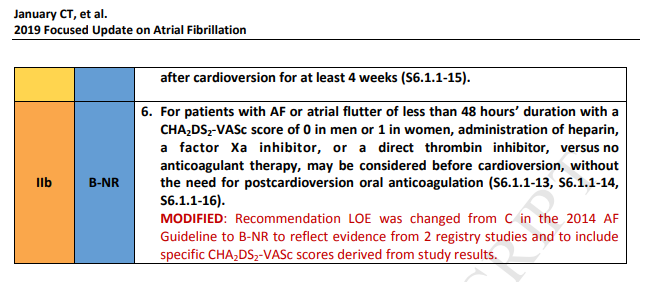
4. Prevention of Thromboembolism
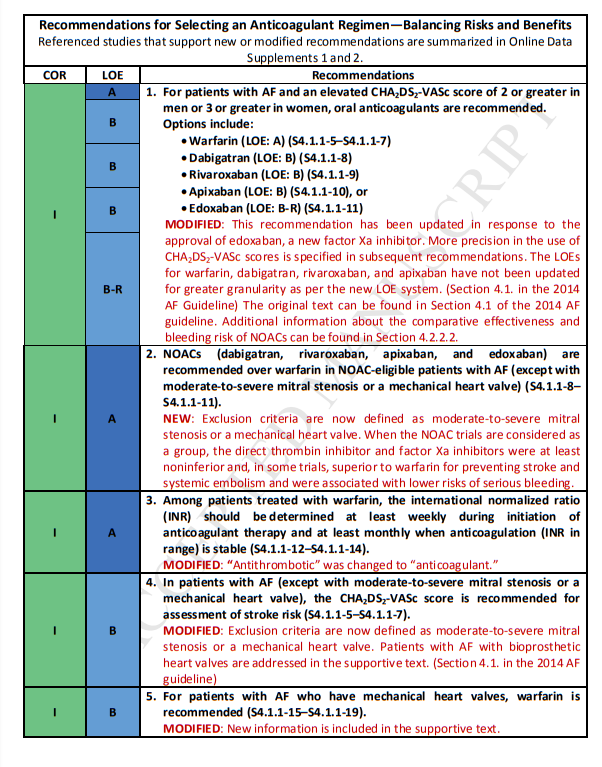
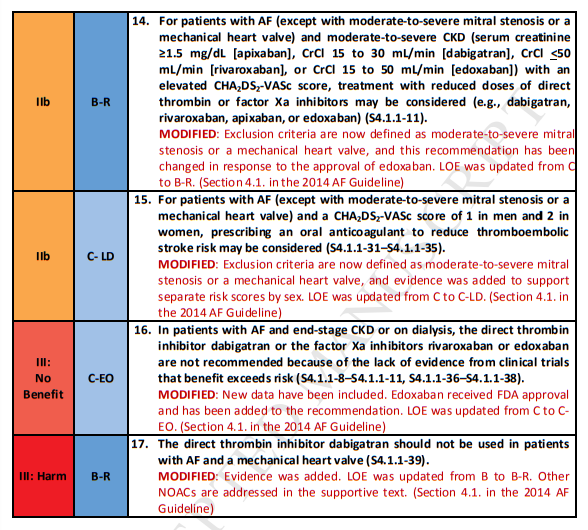
4.1. Risk-Based Anticoagulant Therapy (Modified From Section 4.1., “RiskBased Antithrombotic Therapy,” in the 2014 AF Guideline)
4.1.1. Selecting an Anticoagulant Regimen—Balancing Risks and Benefits (Modified From Section 4.1.1., “Selecting an Antithrombotic Regimen—Balancing Risks and Benefits,” in the 2014 AF Guideline)
Introductory Text
The distinction between nonvalvular and valvular AF has confused clinicians, varying among AF clinical
trials of non–vitamin K oral anticoagulants (NOACs) (i.e., dabigatran [a direct thrombin inhibitor] and
rivaroxaban, apixaban, and edoxaban [factor Xa inhibitors]; also referred to as direct-acting oral anticoagulants [DOACs]) and between North American and European AF guidelines. Valvular AF generally refers to AF in the setting of moderate-to-severe mitral stenosis (potentially requiring surgical intervention) or in the presence of an artificial (mechanical) heart valve. Valvular AF is considered an indication for long-term anticoagulation with warfarin. In contrast, nonvalvular AF does not imply the absence of valvular heart disease. Instead, as used in the present focused update, nonvalvular AF is AF in
the absence of moderate-to-severe mitral stenosis or a mechanical heart valve. This is because in most AF NOAC clinical trials, up to approximately 20% of patients were enrolled with various valvular defects, including mild mitral stenosis, mitral regurgitation, aortic stenosis, aortic regurgitation, and tricuspid regurgitation (S4.1.1-1, S4.1.1-2); some trials enrolled small numbers of patients with valve repair, valvuloplasty, and bioprosthetic valves. Furthermore, meta-analysis–derived data from the original clinical trials suggest that, among patients with AF and these valvular lesions and operations, NOACs reduce stroke and systemic embolism compared with warfarin, but with differences in bleeding risk
(S4.1.1-3). For recommendations from the 2014 AF guideline that were modified only to define the exclusion criteria for valvular AF or to change “antithrombotic” to “anticoagulant,” LOE and supportive text have not been updated. A fifth NOAC, betrixaban, has not been approved by the FDA for use in patients with AF. Antithrombotic (anticoagulant combined with antiplatelet) therapy is discussed in Sections 4.4.1. and 7.4. (S4.1.1-4).
Please see pp 13 – 16 of the PDF Recommendation-Specific Supportive Text (New or Modified) of the above.
4.2. Anticoagulant Options (Modified From Section 4.2., “Antithrombotic Options,” in the 2014 AF Guideline)
4.2.2.2. Non–Vitamin K Oral Anticoagulants (Modified From Section 4.2.2.2., “New Target-Specific Oral Anticoagulants,” in the 2014 AF Guideline)
Synopsis
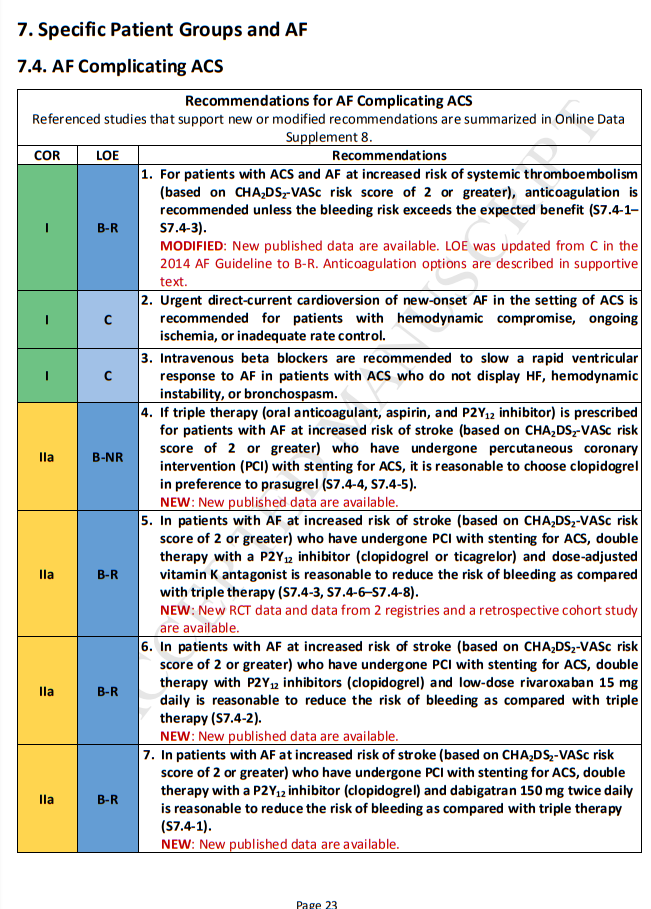
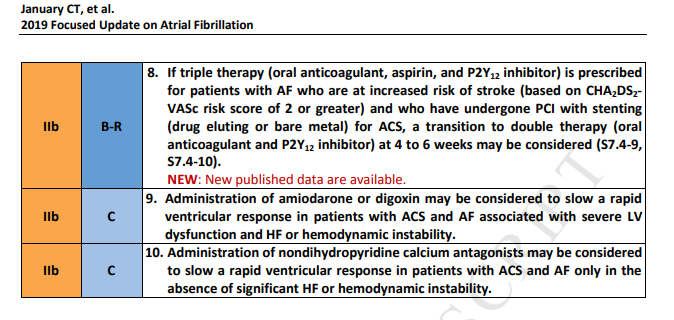
The incidence of AF in patients with ACS ranges from 10% to 21% and increases with patient age and severity of myocardial infarction (MI) (S7.4-11, S7.4-12). In the Medicare population, AF is associated with increased in-hospital mortality rate (25.3% with AF versus 16.0% without AF), 30-day mortality rate
(29.3% versus 19.1%), and 1-year mortality rate (48.3% versus 32.7%) (S7.4-12). With multivariate adjustment, AF remains an independent predictor of death: in hospital (odds ratio: 1.21), at 30 days (odds ratio: 1.20), and at 1 year (odds ratio: 1.34) (S7.4-12). Patients who develop AF during hospitalization have a worse prognosis than those with AF on admission (S7.4-12). Stroke rates are higher in patients with MI and AF than in those without AF (3.1% for those with AF versus 1.3% for those in sinus rhythm) (S7.4-11). Thus, AF is an independent predictor of poor long-term outcome in patients with ACS (S7.4-13, S7.4-14).Patients treated for ACS normally require dual-antiplatelet therapy (DAPT) with aspirin plus a platelet P2Y12 receptor inhibitor and may require the addition of warfarin or a NOAC (“triple therapy”) for primary prevention for patients with AF at increased risk of stroke (S7.4-3) (Section 4.3.). An option is
to consider double therapy—the use of an oral anticoagulant plus a P2Y12 inhibitor without aspirin (S7.4-
3). If triple therapy is used, efforts may be directed to minimize duration of triple therapy to a period of
4 to 6 weeks, as this is the period of greatest risk of stent thrombosis, especially in patients with ACS,
such as ST-segment–elevation MI. Use of DAPT alone may be considered for patients with ACS who have
AF and a CHA2DS2-VASc score of 0 to 1, with reconsideration of the indications for anticoagulation over time (S7.4-15, S7.4-16). Whereas Section 4.1.1. provides specific guidance on the presence/absence of stroke risk associated with female sex in the CHA2DS2-VASc score, the randomized data set referenced in
this section on double versus triple therapy in patients undergoing PCI (subset with ACS) does not
present the data analysis stratified by sex; therefore, the recommendation is provided in the context of overall CHA2DS2-VASc score. The HAS-BLED score can be used to assess bleeding risk in patients for whom anticoagulation is being considered (S7.4-17).Urgent direct-current cardioversion is appropriate in patients with ACS presenting with newonset AF and intractable ischemia, hemodynamic instability, or inadequate rate control. Intravenous administration of a beta blocker is indicated for rate control in patients with ACS to reduce myocardial
oxygen demands. Intravenous amiodarone is an appropriate alternative for rate control and may facilitate conversion to sinus rhythm. Digoxin may be considered in those with severe LV dysfunction and HF or hemodynamic instability. However, recent data from the ARISTOTLE AF NOAC trial study
population show that digoxin was independently associated with higher mortality rate in patients with AF regardless of HF, and in patients with AF taking digoxin, the risk of death increased with higher serum digoxin concentrations (S7.4-18). Other meta-analysis studies support these conclusions (S7.4-19). Treatment with angiotensin-converting enzyme inhibitors appears to reduce the incidence of AF in patients with LV dysfunction after ACS (S7.4-20, S7.4-21).
Recommendation-Specific Supportive Text (New)
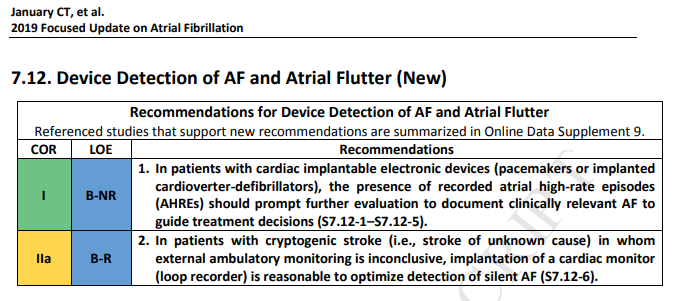
1. Patients with AHREs detected by implanted devices are at increased risk of stroke and abundant data now link device-detected atrial tachycardia or AF (or AHREs) with the development of thromboembolic events (S7.12-1–S7.12-5). Remote monitoring with AHRE alerts increases the likelihood of detecting silent AF. However, it is unclear whether patients with AHREs benefit from oral anticoagulation. Careful review of stored electrograms may confirm the presence of AF and rule
out false positive events. Occasionally, the addition of extended external electrocardiographic monitoring may be needed if data from the implanted device are uncertain. Prospective clinical trials of prophylactic anticoagulation based on device-detected AF are under way but have not been completed. Although increased duration of AHREs is associated with increased stroke risk, the threshold duration of AHREs that warrants anticoagulation is unclear. Current approaches factor in
the duration of device-detected AF and the patient’s stroke risk profile, bleeding risk, and preferences to determine whether to initiate long-term anticoagulatio2. The cause of ischemic stroke remains unknown in 20% to 40% of patients, leading to a diagnosis of cryptogenic stroke. Prolonged electrocardiogram monitoring with an implantable cardiac monitor in these patients (age >40 years) has the advantage of increasing the likelihood of detecting silent AF that would escape detection with short-term monitoring. A recent RCT established the superiority of an implantable cardiac monitor over conventional monitoring for detecting silent AF, a finding with major clinical ramifications for these patients (S7.12-6). A role in screening for silent AF may also exist for remote electrocardiographic acquisition and transmission with a “smart” worn or handheld WiFi-enabled device with remote interpretation (S7.12-7, S7.12-8).
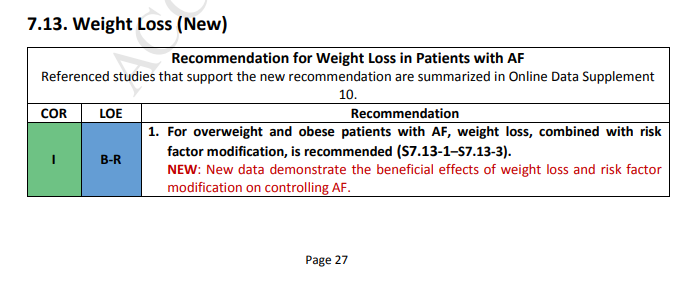
Recommendation-Specific Supportive Text (New)
1. Obesity is associated with atrial electrostructural remodeling (S7.13-4) and AF (S7.13-5–S7.13-7).
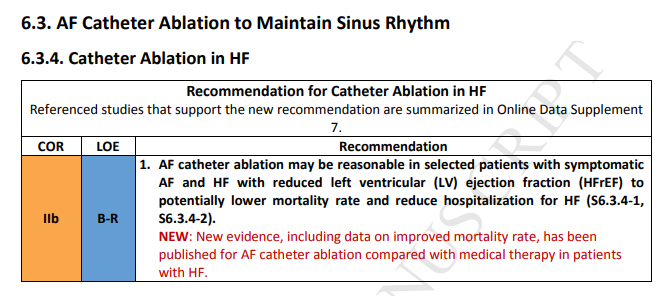
One RCT demonstrated that a structured weight management program for obese patients (body mass index >27) with symptomatic AF reduced symptom burden and severity and reduced the number of AF episodes and their cumulative duration when compared with attempts to optimally manage risk factors alone (S7.13-1). Risk factor modification included assessment and treatment of underlying sleep apnea, hypertension, hyperlipidemia, glucose intolerance, and alcohol and tobacco use. A second nonrandomized observational study reported improved outcomes of AF catheter ablation among obese patients who enrolled in a weight loss program (S7.13-2). Observational studies have revealed that the degree of improvement in the AF type and symptoms were related to
the degree of weight loss (S7.13-3, S7.13-8). Taken together, these studies support a treatment approach that addresses the risk factors for AF.