In addition to today’s resource, please see Linking To And Embedding The YouTube Video, “POCUS When You Can’t Poop: The Ultrasound Evaluation From MetroHealth Emergency Ultrasound of Small Bowel Obstruction” From MetroHealth
Posted on September 11, 2022 by Tom Wade MD
In today’s post, I link to and excerpt from Imaging Modalities for Evaluation of Intestinal Obstruction [PubMed Abstract] [Full-Text HTML] [Full-Text PDF].
Nelms, D. W., & Kann, B. R. (2021). Bowel Obstructions: Imaging Modalities for Evaluation of Intestinal Obstruction. Clinics in Colon and Rectal Surgery, 34(4), 205-218. https://doi.org/10.1055/s-0041-1729737
All that follows is from the above resource.
Abstract
It is essential for the colon and rectal surgeon to understand the evaluation and management of patients with both small and large bowel obstructions. Computed tomography is usually the most appropriate and accurate diagnostic imaging modality for most suspected bowel obstructions. Additional commonly used imaging modalities include plain radiographs and contrast imaging/fluoroscopy, while less commonly utilized imaging modalities include ultrasonography and magnetic resonance imaging. Regardless of the imaging modality used, interpretation of imaging should involve a systematic, methodological approach to ensure diagnostic accuracy.
Keywords: bowel obstruction, small bowel obstruction, large bowel obstruction, imaging, computed tomography, abdominal radiography, contrast enema, small bowel follow-through, ultrasound, magnetic resonance imaging
Intestinal obstruction is a clinical scenario commonly encountered by colorectal surgeons, 1 and it is important to understand the evaluation and management of patients with both suspected small bowel obstruction (SBO) and large bowel obstruction (LBO). Approximately 75% of all mechanical bowel obstructions occur in the small bowel. 2 3 SBO occurs in 10% of patients within 3 years following colectomy 4 and in up to 25% of patients after restorative proctocolectomy. 5 Multiple colorectal surgery–related pathologies can result in SBO, including postoperative adhesions, Crohn’s disease, diverticulitis, and parastomal hernias, among others. Evaluation and management of LBO can be a complex problem that challenges even the most experienced clinicians. 6 Common etiologies of both SBO and LBO are listed in Table 1 .
Diagnostic imaging is an essential aspect of the modern management of both LBO and SBO. While history and physical exam remain the backbone of evaluation, clinical assessment alone lacks accuracy for bowel obstruction diagnosis and guidance of management. 7 8 9 Imaging helps answer a variety of key questions in patients with suspected obstruction, including the following: 10 11
This article will review the available modalities for the evaluation of suspected mechanical intestinal obstruction in the context of the three questions above.
Plain Radiographs
Traditionally, plain abdominal radiographs have been recommended as the initial imaging modality for suspected obstructions due to the speed of acquisition, low cost, wide availability, and low radiation exposure.
However, the accuracy of plain radiographs in the diagnosis of bowel obstruction ranges from only 50 to 80%. 12 Additionally, in only a minority of cases do plain abdominal radiographs provide a clear etiology of an obstruction. Plain radiographs are poor at identifying closed loop or strangulated obstructions in the setting of SBO, and the specificity of plain radiographs for LBO is only moderate, in part due to mimicry of acute colonic pseudoobstruction causing false positives. 13 Therefore, even when plain abdominal radiographs appear to definitively establish a diagnosis, obtaining additional information via computed tomography (CT) is often still necessary.
Computed Tomography
CT accuracy for the diagnosis of both SBO and LBO is greater than 95%. 28 29 30 This has led some to question the traditional approach of using of plain radiographs as the initial imaging modality in the evaluation of patients with suspected bowel obstruction. 8 31 The “Appropriateness Criteria for Suspected Small-Bowel Obstructions,” published by the American College of Radiology (ACR), states that for initial imaging of the patient with suspected SBO, CT abdomen/pelvis is “usually appropriate” while plain radiographs “may also be appropriate.” 32 The use of a multidetector CT is essential, as multiplanar reconstructions and review of axial, coronal, and sagittal images have been shown to increase the accuracy of interpretation. 33 34
When CT is performed to evaluate suspected bowel obstruction, intravenous (IV) iodinated contrast should be administered unless contraindicated. 32 35 The use of IV contrast has not been shown to significantly change the sensitivity of CT for the detection of bowel obstructions, but it can improve assessment for bowel wall ischemia. 36 Enteric contrast should also be administered in certain clinical scenarios. Enteric contrast agents used for CT can be categorized as positive (radiodensity > water), neutral (radiodensity ≈ water), and negative (radiodensity < water; e.g., gas). Most enteric contrast used for CT is water-soluble iodinated-based contrast but dilute barium preparations for CT do exist. 37 Standard barium cannot be used for CT due to its high radiodensity that creates artifacts obscuring images.
Indications for administration of enteric contrast for CT evaluation of suspected bowel obstructions differ depending on the clinical suspicion. In cases of a suspected high-grade SBO, oral contrast prior to CT should generally be avoided. In this setting, retained fluid within the distended small bowel acts as a natural neutral contrast agent allowing for the same diagnostic accuracy as with oral contrast. 36 In fact, positive oral contrast administration may actually decrease the ability to assess for bowel wall ischemia in this setting. 32 The aforementioned ACR Appropriateness Criteria state that “oral contrast used in a known or suspected high-grade SBO does not add to diagnostic accuracy and can delay diagnosis, increase patient discomfort, and increase the risk of complications, particularly vomiting and aspiration.” 32
In patients with indolent or chronic intermittent obstructive symptoms, the use of large volume neutral contrast administered orally or via a nasoenteric tube can also improve accuracy as part of a specific protocol.
In cases of suspected LBO, there is a paucity of published data regarding whether oral and/or rectal contrast should be administered, 29 and no apparent society guidelines exist. Administration of oral contrast alone has the disadvantage of a prolonged waiting period prior to opacifying the colon. While rectal contrast may increase patient discomfort, it may help delineate the site of an obstruction or more definitively rule out mechanical obstruction. Rectal contrast should not be administered if there is any suspicion for perforation. If initial CT is obtained without rectal contrast and diagnosis is in question or further clarity regarding the lesion is needed, CT can be repeated with water-soluble rectal contrast 13 without repeat IV contrast or a water-soluble contrast enema can be obtained.
Is Obstruction Present and at What Level?
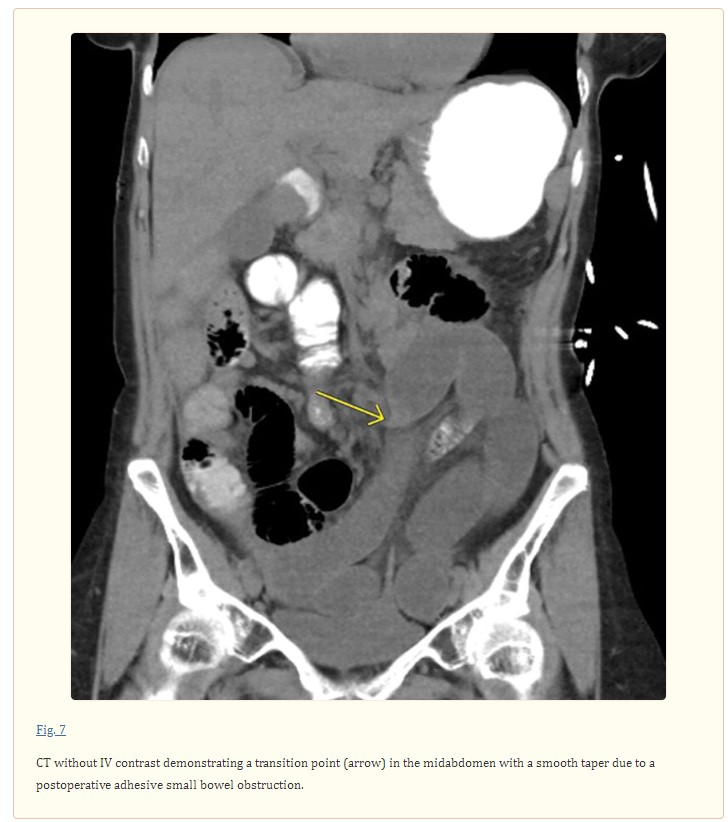
The major diagnostic finding for bowel obstruction on CT is proximal dilation with distal decompression. Intestinal dilation on CT is considered to be present at a small bowel diameter greater than 2.5 cm, colon diameter greater than 6 cm, and cecal diameter greater than 9 cm. 11 38 39 In addition to providing high diagnostic accuracy, cross-sectional imaging often allows precise anatomic localization of the site of obstruction by the identification of a transition zone (TZ) where dilated proximal bowel transitions to nondilated distal bowel ( Fig. 7 ).
Review of abdominopelvic CT for suspected bowel obstruction should be performed systematically. One proposed method is to begin at the anus and trace the bowel proximally. 38 40 This allows for complete inspection of the large bowel and early identification of LBO, particularly when there is concomitant small bowel dilation due to an incompetent ileocecal valve. In the setting of LBO, identification of a TZ on CT is typically possible, while the ability to identify a distinct TZ in the setting of SBO is less reliable, with reported ranges of 63 to 93%. 11 In instances of a more proximal SBO, identification of a TZ can be aided by tracing antegrade starting from the stomach. 38 It should be noted that the ability to detect a clear TZ in SBO is not absolutely necessary for diagnosis, and the ability to identify a TZ does not necessarily correlate with outcome. 41
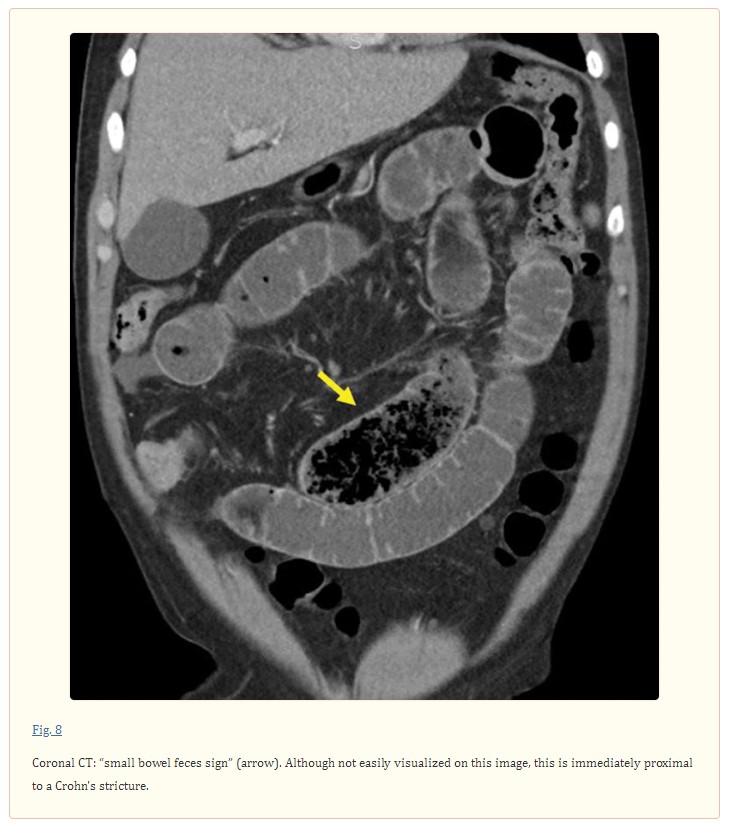
Localizing a TZ in the setting of SBO can be aided by identification of a “small bowel feces sign” or “small bowel fecalization,” which is seen when a mixture of intraluminal particulate material and gas bubbles within the small bowel creates an appearance on CT similar to that colonic stool ( Fig. 8 ). First described by Mayo-Smith, 42 this finding has been ascribed various levels of clinical importance. Initial reports described its presence as uncommon (∼7%) 43 on CT images diagnostic for SBO, while later reports have reported it to be present in 37 to 55.9% of patients with CT evidence of SBO. 44 45 This finding can help readily identify a TZ; it has been reported that in 93% of cases in which the “feces sign” is present in the setting of SBO, it is seen immediately proximal to the TZ. Of note, the administration of positive oral contrast may obscure this finding.
As with plain radiographs, there are several important mimics of SBO and LBO on CT. Low-grade SBO without a distinct TZ can mimic the appearance of an adynamic ileus. Spasm at any level of the normal colon, particularly the flexures and sigmoid colon, can mimic a fixed narrowing, giving the appearance of LBO. 46 Acute and chronic colonic pseudoobstruction can also cause proximal dilation with distal decompression. In the setting of pancreatitis, isolated gaseous distension of the ascending colon and hepatic flexure (termed the “colon cutoff sign”) can also mimic LBO. 47
What Is the Cause of Obstruction?
Small Bowel Obstruction
As previously discussed, approximately 75% of SBOs are caused by adhesions. On CT imaging, the diagnosis of adhesive SBO is presumptive, based on the presence of obstruction, the patient’s clinical history, and exclusion of other findings. Careful study of the TZ is essential for identifying a potential etiology of SBO on CT, as radiographic clues will often be immediately in proximity to the TZ. Many nonadhesive etiologies of SBO can also be identified on CT, including hernia (external and internal), small bowel masses, gallstones, strictures (e.g., related to Crohn’s disease), intussusception, and small bowel volvulus, or closed loop obstruction. Benign small bowel masses such as lipomas and gastrointestinal stromal tumors causing obstruction can often be identified on CT. Small bowel primary malignancies are rare but can sometimes be identified as masses at the TZ. Small intestinal or ileocecal intussusception can often be visualized as a “target sign.”
Large Bowel Obstruction
CRC is the cause of greater than 60% of LBO, 3 13 with more than 75% of obstructing CRCs occurring distal to the splenic flexure. CT findings suggestive of CRC include an enhancing soft-tissue mass or short-segment asymmetric thickening of the colonic wall occurring at the TZ. 22 Diagnostic suspicion for CRC may also be heightened by identification of associated lymphadenopathy or lesions in the liver or lungs suspicious for metastatic disease. Colonoscopy and direct visualization/biopsy of the colonic lesion is necessary for a definitive diagnosis of CRC, though this is not always possible prior to surgical intervention in the patient who presents with complete or near-complete LBO and impending perforation.
Diverticulitis is responsible for approximately 3.6 to 10% of LBO. 3 22 In comparison to LBO due to CRC, these lesions typically appear on CT as longer, more symmetric segmental colonic thickening at the TZ. 22 Associated findings with active disease include mesenteric fat stranding, abscess, and phlegmon, but these findings are often difficult to differentiate from perforated CRC, highlighting the need for follow-up colonoscopy following resolution of an episode of acute diverticulitis. 48 Additional CT findings with chronic diverticular disease include progressive colonic wall thickening, fibrosis, and stricture formation. The presence of air in the bladder or vagina associated with a chronically diseased segment of sigmoid colon on CT suggests the presence of a colovesical or colovaginal fistula.
Diverticulitis is responsible for approximately 3.6 to 10% of LBO. 3 22 In comparison to LBO due to CRC, these lesions typically appear on CT as longer, more symmetric segmental colonic thickening at the TZ. 22 Associated findings with active disease include mesenteric fat stranding, abscess, and phlegmon, but these findings are often difficult to differentiate from perforated CRC, highlighting the need for follow-up colonoscopy following resolution of an episode of acute diverticulitis. 48 Additional CT findings with chronic diverticular disease include progressive colonic wall thickening, fibrosis, and stricture formation. The presence of air in the bladder or vagina associated with a chronically diseased segment of sigmoid colon on CT suggests the presence of a colovesical or colovaginal fistula.
Colonic volvulus most commonly occurs at the level of the sigmoid colon (70%), followed by the cecum (25%) and transverse colon (5%). 21 On CT imaging, the segment of redundant colon is often massively elongated and dilated, and is typically associated with twisting of the mesocolon, known as a “whirl sign” ( Fig. 9 ). The proximal and distal limbs of the loop can also be seen to taper in a “bird’s beak” fashion. Additional CT signs of colonic volvulus include the “X-marks-the-spot” sign which is the visualization of two TZs crossing in opposite directions at the same location, and the “split-wall” sign in which the colonic wall appears separated by mesenteric fat due to folding occurring with the twist. 49 50
Additional causes of LBO are much less common, but still occasionally seen on CT imaging. Colocolonic intussusception is rare, and most frequently occurs due to colonic neoplasm. CT signs of intussusception includes the “target sign” when viewed perpendicular to the lumen and the “sausage pattern” when viewed parallel to the lumen 22 ( Fig. 10A, B ). Fecal impaction, most commonly in the rectosigmoid, can result in LBO. This diagnosis should be considered when focal stool is present that is equal to or greater than the upstream colonic diameter without any associated soft-tissue mass. Inflammatory bowel disease can also cause LBO as a result of colonic strictures. 49 Crohn’s disease causes strictures of the colon more frequently than ulcerative colitis due to the transmural nature of inflammation. On CT, IBD-related strictures typically appear as focal colonic wall thickening with enhancement and can be difficult to differentiate from CRC, again highlighting the need for endoscopic visualization and biopsy.
Is Severe or Complicated Obstruction Present?
CT is also particularly useful for assessing the severity of obstruction (partial vs. complete or low-grade vs. high-grade) and for identifying potential complications that can guide decisions regarding the need and timing for operative intervention. When positive oral contrast is administered, failure of contrast to progress past the TZ in 3 to 24 hours can be diagnostic of complete obstruction. 38 51 However, oral contrast is often either not administered or insufficient time has elapsed at the time of imaging to allow passage of contrast for definitive diagnosis of complete obstruction. 51 In this scenario, a subjective judgment of the severity of obstruction can be made. Factors used to grade the severity of SBO include a subjective grading of the quantity of gas/fluid in the distal small bowel and/or the ascending colon, and quantitative comparison of the proximal and distal luminal diameters, with a difference of ≥50% felt to be suggestive of high-grade obstruction.38 Terminology can be ambiguous, with the term “high-grade” often being used synonymously with “complete obstruction,” while partial obstructions are frequently subcategorized into high-grade partial and low-grade partial obstruction.
Complications related to intestinal obstruction that can be identified on CT include perforation, closed loop obstruction, internal hernia, volvulus, and intestinal ischemia. These are frequently identified only after careful inspection of the TZ on multiple reconstructed CT views. 38 CT allows for extremely sensitive identification of pneumoperitoneum and free fluid. Free intraperitoneal fluid is present in over one-third of patients with acute SBO and is nonspecific for need of operative intervention. In one study, fluid with a Hounsfield unit density greater than 10 was associated with a positive predictive value and negative predictive value of ≥75% in predicting the need for operative intervention. 52
Closed loop SBO can be identified on CT but requires a high index of suspicion. Closed loop SBO often results from a single constricting lesion such as an adhesive band that occludes both proximally and distally and can be associated with volvulus. 11 Another common etiology of closed loop SBO is an internal hernia caused by congenital or iatrogenic mesenteric defects. Knowledge of the patient’s surgical history is essential in evaluating imaging studies in the setting of suspected intestinal obstruction. Patients who have undergone prior Roux-en-Y gastric bypass are at risk for SBO due to internal hernia, with potential sites of mesenteric defects at the jejunojejunostomy, transverse mesocolon (if a retrocolic roux limb was created), and beneath the gastrojejunostomy (Peterson’s hernia). On CT, a closed loop obstruction or volvulus typically appears as a “C” or “U” with the concavity pointing to the site of obstruction, when the loop is within the plane of imaging. At the site of tethering, the ends of the loop narrow to a “beak” configuration. When there is coexistent volvulus, the mesentery will be twisted and the “whirl sign” can be present similar to colonic volvulus. 11 It is important to assess the images in multiple reconstructed views, as a closed loop may sometimes be most apparent on a coronal or sagittal view. 11
CT is highly specific for the detection of intestinal ischemia, but it lacks sensitivity. Therefore, one must have a high index of suspicion for intestinal ischemia based on the patient’s clinical presentation. Radiographic signs of bowel ischemia are listed in Table 3 . In one study that compared prospective and retrospective review of CT for the detection of intestinal ischemia, the prospective interpretation showed a sensitivity of only 14.8% and a specificity of 94.1%. When the imaging studies were re-reviewed retrospectively by two additional blinded, independent gastrointestinal radiologists, the sensitivity was still only 51.9% and the specificity was 88.2%. 53 The administration of IV contrast can increase the sensitivity of ischemia detection by demonstrating relative hypoenhancement or heterogenous enhancement of strangulated bowel compared with adjacent nonischemic bowel. In noncontrast CT images, hemorrhagic infarcted or ischemic bowel will often appear hyperattenuated. 11 Portal venous gas on CT is a particularly worrisome finding in the setting of intestinal obstruction. Portal venous gas should not be confused with pneumobilia which can occur with gallstone-related obstruction from a cholecysto-enteric fistula, as well as in patients who have undergone recent ERCP or prior surgery with a biliary-enteric anastomosis. Portal venous gas appears in a tubular and branching pattern extending peripherally (within 2 cm of the liver capsule), whereas pneumobilia appears as isolated bubbles and is located centrally (> 2 cm from the capsule). 54
Contrast Imaging/Fluoroscopy
While CT and plain X-ray are typically the most appropriate initial imaging modalities for patients with suspected bowel obstruction, contrast imaging/fluoroscopy studies are important common adjuncts that can help clarify specific clinical questions and guide therapeutic intervention.
Water-Soluble Contrast Enema
The use of the fluoroscopic unprepped contrast enema historically played a significant role in the initial diagnosis of acute LBO. 55 However, with the advent and wide availability of multidetector CT, the indications for contrast enema are now more limited and it is largely reserved for complimenting CT findings. 13 22 28 55 A review by Jacob et al 55 demonstrated a one-third decrease in contrast enema use for the evaluation of suspected LBO from the years 2000–2006 with a concomitant increase in the use of multidetector CT for the same indication.
Contemporary indications for diagnostic contrast enema include evaluation of equivocal cases of LBO, where it can help distinguish LBO from acute colonic pseudo-obstruction, 13 56 and the evaluation of equivocal cases of colonic volvulus. 13 In the setting of suspected LBO, the examination should be performed with water-soluble contrast, with low pressure, and without inflation of the catheter balloon. 13 Barium should be avoided in the setting of suspected LBO because of the risk of intraperitoneal contamination with perforation and the potential interference with subsequent cross-sectional imaging or colonoscopy. 37 Complete examination does require patient rotation on the fluoroscopy table and therefore the exam can be limited in patients who are unable to participate in these maneuvers. 13
Small Bowel Contrast Studies
Formal small bowel follow-through (SBFT) is usually not performed as an initial test for acute obstruction, but it has a role in the evaluation of low-grade and chronic SBOs. 11 37 SBFT is performed with barium because water-soluble contrast is inadequate for delineation of small bowel anatomy and dilution of the contrast within the small bowel with progression typically does not allow identification of a more distal TZ. 37 59 Barium can be safe to administer in suspected low-grade SBO when there is no suspicion of perforation, but it should be avoided in high-grade SBO. 37 Barium can also interfere with subsequent cross-sectional imaging by creating artifact. It is important to rule out LBO prior to administration of oral barium because when barium is trapped proximal to a colonic lesion, progressive water absorption can lead to barium inspissation (“barolith”) with risk of worsening obstruction, ulceration, ischemia, and perforation. 60
Enteroclysis is similar to SBFT except that instead of oral contrast administration, enteral contrast is rapidly administered via a postpyloric enteric tube. Contrast media include singular or combination barium, methylcellulose, and/or air. Enteroclysis has greater sensitivity than SBFT for subtle obstructing lesions as a result of over distending the bowel proximal to a lesion. This examination is less commonly performed than SBFT due to both patient discomfort and logistics of nasoenteric tube placement.
Conclusion
Imaging plays a central role in the modern evaluation and management of suspected intestinal obstruction. CT is the imaging modality of choice for the majority of patients with suspected intestinal obstruction, as it is practical to obtain, accurate for diagnosis, and provides substantial information to help determine the cause of obstruction and identify complications. Plain radiographs may serve as an initial screening modality and can be useful for serial imaging examinations in the setting of nonoperative management. Contrast enema and SBFT are important adjuncts to CT for equivocal cases. Water-soluble oral contrast challenge for adhesive SBO is a tool that can predict those who will succeed nonoperative management and potentially reduce length of hospital stay. Ultrasound is useful in children and pregnant women, and POCUS is an alternative screening exam for adults. MRI is an alternative cross-sectional modality that can exceed the accuracy of CT, but practical considerations significantly limit its use. The routine and systematic review of all radiographic images by the surgeon may improve patient outcomes and improve the surgeon’s skill at image interpretation in the evaluation of the patient with suspected intestinal obstruction.