This post contains my study notes for the sections of the 2018 Guidelines that pertain to revascularization of stable coronary artery disease. This portion of the 2018 Guidelines runs from pp 8 – 24 and it is worth reviewing all of the pages. [Note to myself]
Any patient presenting to an urgent care center or primary care office with an acute coronary syndrome will most likely be immediately transferred by paramedic ambulance to an emergency department.
Since this post is directed at primary care office based practitioners [like me], I have only included the first page of the Table Of Contents [as it includes Diagnostic tools, Process for decision making, and Revascularization for stable coronary artery disease – the knowledge base most relevant to primary care].
See Resource (1) below for direct links to the entire 2018 ESC/EACTS Guidelines on myocardial revascularization [which covers all the relevant clinical scenarios.
And here are some excerpts from the article:
2 Introduction
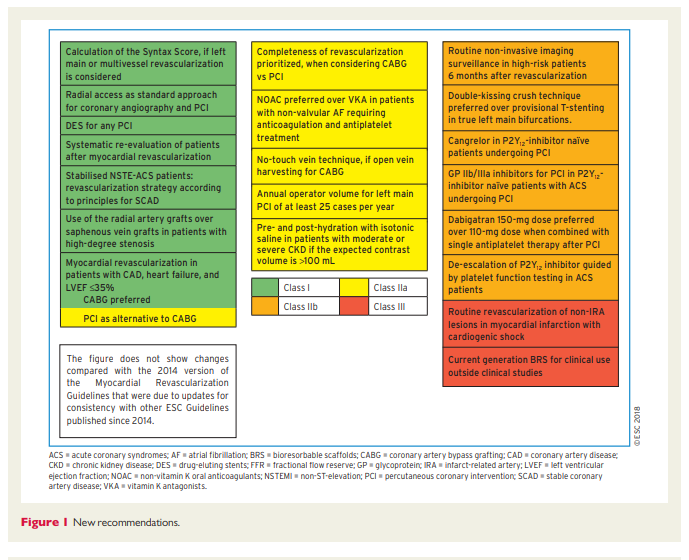
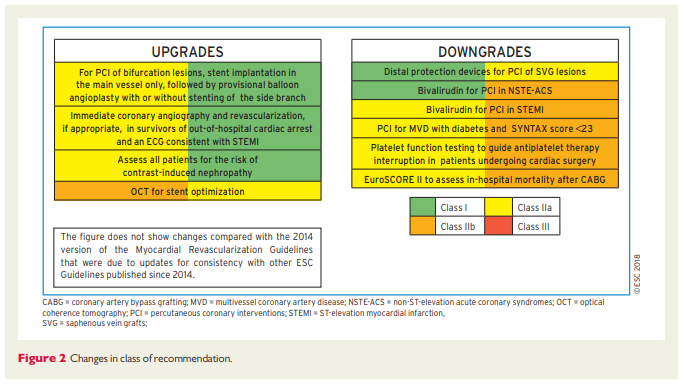
These Guidelines represent the third time that the ESC and EACTS have brought together cardiologists and cardiac surgeons in a joint Task Force to review the ever-increasing body of evidence, with the mission of drafting balanced, patient-centred practice Guidelines on myocardial revascularization. Summaries of the key changes in comparison with the previous Guidelines are provided in Figures 1 and 2.
2.1 What is new in the 2018 Guidelines?
3 Diagnostic tools to guide myocardial revascularization
The use of diagnostic imaging and functional testing modalities to detect patients with coronary artery disease (CAD) is discussed in detail in the clinical practice Guidelines for patients with SCAD. [Resource (4) below] Further diagnostic assessment of patients with obstructive CAD is critical in order to identify patients and select specific lesions that are likely to benefit from myocardial revascularization, in addition to optimal medical therapy.
3.1 Non-invasive diagnostic tools
3.1.1 Assessment of myocardial ischaemia
Non-invasive diagnostic assessment of patients with CAD being considered for myocardial revascularization comprises the assessment of ischaemia and the evaluation of viability in patients with regional wall motion abnormalities or reduced ejection fraction (EF).
Functional testing to assess ischaemia is critical for the assessment of stable patients with CAD. Documentation of ischaemia using functional testing before elective invasive procedures for CAD is the preferred approach. It may also have a role in the assessment of some patients presenting with acute coronary syndrome (ACS). Because of the low sensitivity of exercise electrocardiogram (ECG) testing in the
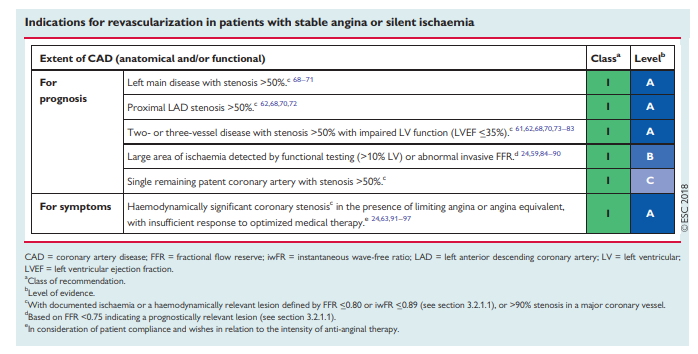
assessment of patients with symptoms of angina, non-invasive imaging is recommended as the first-line test.1 Detection of a large area of myocardial ischaemia by functional imaging is associated with impaired prognosis of patients and identifies patients who should undergo revascularization (see section 5).In patients undergoing coronary computed tomography (CT),
both CT-derived fractional flow reserve (CT-FFR) and CT perfusion represent possible approaches to evaluate lesion-specific ischaemia. Although the evidence for both is limited at present, there are considerably more data from clinical investigations of CT-FFR. A number of trials have shown that correlation between CT-derived FFR and invasive FFR is high.2,3 The non-randomized PLATFORM (Prospective LongitudinAl Trial of FFRct: Outcome and Resource Impacts) study showed that in patients referred for invasive angiography due to chest pain (predominantly atypical angina) and intermediate pre-test probability of CAD, assessment with CT and CT-FFR reduced the number of patients with subsequently normal invasive coronary angiograms compared with standard care.4 Currently, clinical trial data with CT-FFR are insufficient to make a recommendation for its use in clinical practice.3.1.2 Assessment of myocardial viability in patients with heart failure and coronary artery disease
In patients with regional wall motion abnormalities or ventricular dysfunction, heart failure (HF) can be caused by stunned or hibernating
myocardium and may be reversed by revascularization. Assessment of myocardial viability may be done in order to select patients that are more likely to benefit from myocardial revascularization and can be achieved with several imaging modalities: myocardial contrast echocardiography, single-photon emission CT (SPECT), and late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) all assess cellular integrity; positron emission tomography (PET) assesses cellular metabolism; and dobutamine techniques assess contractile reserve.1,5 Assessment of ischaemia provides incremental
benefit over viability in mild to moderate CAD, but with extensive CAD viability assessment may be sufficient.6 Patients with advanced HF and viable myocardium should first undergo revascularization with coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) before being considered for mechanical circulatory support (MCS) or heart transplantation.7,83.2.1.2 Use of fractional flow reserve to identify lesions requiring revascularization in patients with multivessel coronary artery disease undergoing percutaneous coronary intervention
FFR may also be useful for the selection of lesions requiring revascularization in patients with multivessel CAD. The FAME trial showed that in patients with multivessel disease randomized to an FFR-guided PCI strategy (using a cut-off <_0.80 to indicate requirement for PCI), outcomes at 12 months in terms of death, non-fatal MI, and repeat revascularization were superior compared with angiography-guided PCI and utilized fewer resources.29 In addition, the 2 year composite risk of death or MI was significantly lower with the FFR-guided PCI strategy.30 Long-term follow-up at 5 years showed broadly consistent findings, although differences between groups in relation to the primary endpoint were no longer significant.31 This suggests that FFR-guided PCI should be the preferred management strategy in these patients.
3.2.2 Other pressure-derived indices
FFR evaluation requires maximal and stable hyperaemia, which is usually obtained by the administration of intravenous (i.v.) adenosine. Recently, there has been renewed interest in resting indices derived from resting gradients alone [distal coronary to aortic pressure (Pd/Pa) or instantaneous wave-free ratio (iwFR)]. Two recent large-scale randomized trials showed broadly comparable results between FFRguided and iwFR guided revascularization strategies in patients with intermediate-grade stenosis.17,18 Revascularization was indicated in both trials if FFR was <_0.80 or if iwFR was <_0.89. In the DEFINEFLAIR trial, the primary endpoint of MACE at 1 year occurred i6.8% in patients randomized to iwFR-guided revascularization vs.7.0% in patients randomized to FFR-guided revascularization (P<0.001 for non-inferiority; HR 0.95, 95% CI 0.68–1.33, P = 0.78).17 In the iFR-SWEDEHEART trial, the primary endpoint of death from any cause, non-fatal MI, or unplanned revascularization was 6.7% in the iwFR group and 6.1% in the FFR group (P = 0.007 for non-inferiority; HR 1.12, 95% CI 0.79–1.58, P = 0.53).18 In this trial, 17.5% of patients had ACS at the time of presentation. There was no interaction with outcomes. Both trials are limited by having a follow-up duration of only 1 year.
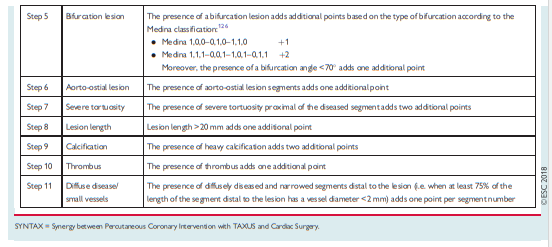
The SYNTAX II study (Synergy between Percutaneous Coronary
Intervention with TAXUS and Cardiac Surgery), a single-arm, prospective study in patients with multivessel disease incorporating a management strategy including combined iwFR/FFR assessment of stenosis severity in addition to intravascular ultrasound (IVUS)-guided stent implantation and guideline-directed medical therapy, showed encouraging outcomes compared with a historical cohort enrolled in the SYNTAX trial.34Randomized trials comparing iwFR-guided revascularization with angiography-guided revascularization or medical therapy are not available. iwFR has not been extensively validated for patients with LMS stenosis.
There is no adequate randomized controlled trial (RCT) data to
support the use of whole-cardiac cycle Pd/Pa for the guidance of
revascularization decisions.
3.2.3 Use of fractional flow reserve and pressure-derived indices in patients with severe aortic stenosis
See p. 12 of the article
3.2.4 Use of intravascular imaging for the diagnostic
assessment of stenosisSee p. 12 of the article
4. Epidemiology
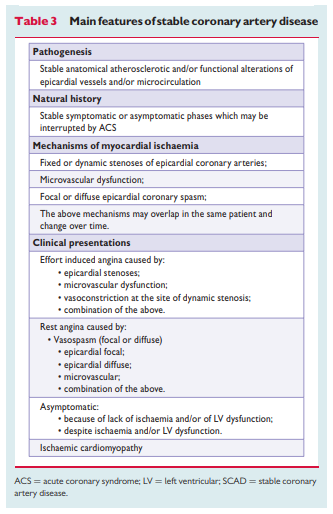
As SCAD is so multifaceted, its prevalence and incidence have been difficult to assess and numbers vary between studies, depending on the definition that has been used. For epidemiologic purposes, stable angina is essentially a diagnosis based on history and therefore relies on clinical judgement. The Rose angina questionnaire has a specificity of 80–95%,12 but its sensitivity varies substantially from 20–80% when compared with clinical diagnosis, ECG findings and coronary angiography.
The prevalence of angina in population-based studies increases
with age in both sexes, from 5–7% in women aged 45–64 years to 10–12% in women aged 65–84 and from 4–7% in men aged
45–64 years to 12–14% in men aged 65–84.13 Interestingly, angina is more prevalent in middle-aged women than in men, probably due to the higher prevalence of functional CAD—such as microvascular angina—in women,14,15 whereas the opposite is true in the elderly.Available data suggest an annual incidence of uncomplicated angina pectoris of 1.0% in male western populations aged 45–65 years, with a slightly higher incidence in women under the age of 65.13,16 There is a steep increase with age and the incidence in men and women 75–84 years of age reaches almost 4%.16 The incidence of angina varies in parallel with observed international differences in CAD mortality.16,17
Temporal trends suggest a decrease in the annual death rate due to CAD.18 However, the prevalence of a history of diagnosed CAD does not appear to have decreased, suggesting that the prognosis of those with established CAD is improving. Improved sensitivity of diagnostic tools may additionally contribute to the contemporaryhigh prevalence of diagnosed CAD.
Epidemiological data on microvascular angina and vasospastic
angina are missing. However, recent clinical data suggest that abnormal coronary vasomotion is present in two-thirds of patients who suffer from stable angina but have no coronary stenoses at angiography.19
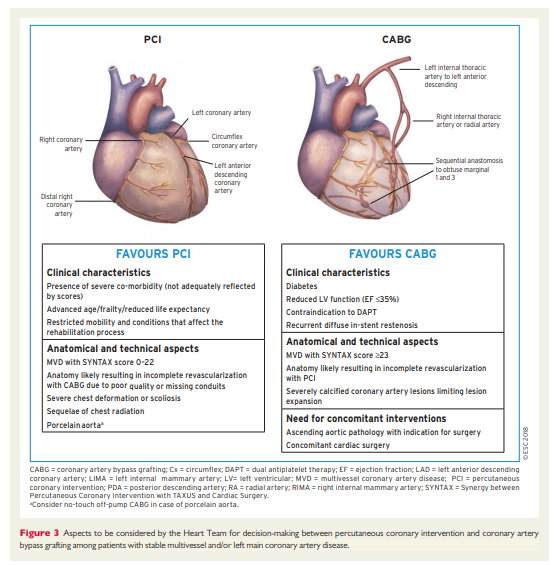
4.2 Multidisciplinary decision-making (Heart Team)
The Heart Team—comprising clinical or non-invasive cardiologists, cardiac surgeons, and interventional cardiologists, as well as anaesthetists and other specialists if deemed necessary—should provide a balanced, multidisciplinary decision-making process.43
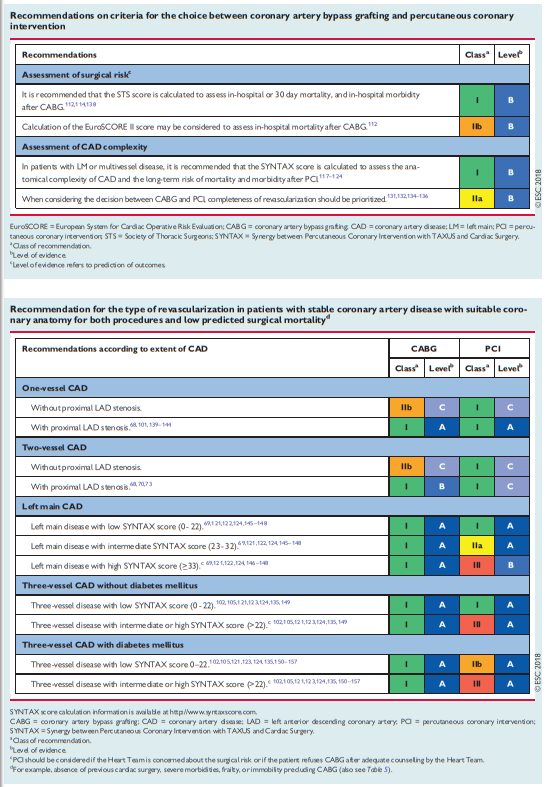
The need for an interdisciplinary approach is underlined by reports on (i) the underuse of revascularization procedures in 18–40% of patients with CAD44 and (ii) inappropriate use of revascularization strategies with a lack of case discussions.45 The marked variability in PCI-to-CABG ratios between European countries (ranging from 2.4–7.6 in 2013, for example) has raised concerns regarding the selection of revascularization strategies.46 Rates for the inappropriate use of PCI (10–15%)43,47,48 and CABG (1–2%) are reported. Multidisciplinary decision-making in a Heart Team can minimize specialty bias and prevent self-referral from interfering with optimal patient care.49
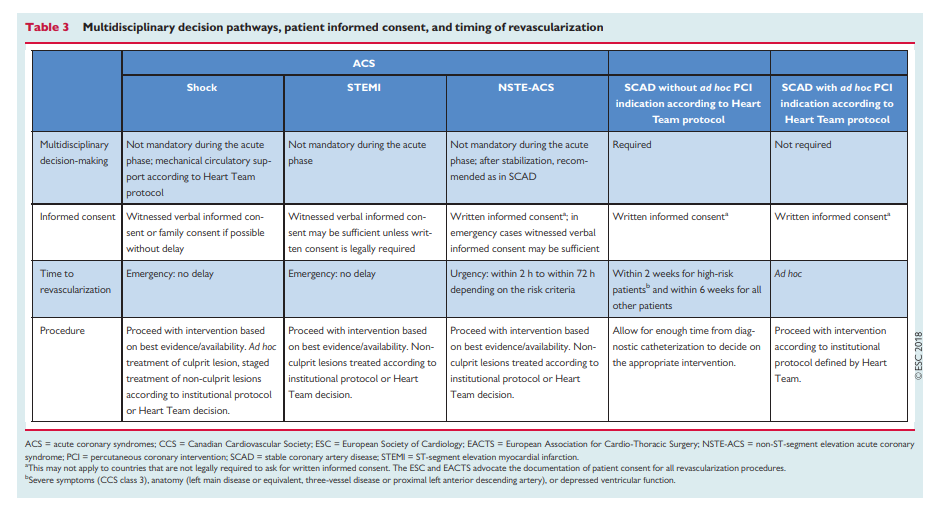
4.3 Timing of revascularization
Patients requiring myocardial revascularization may be at increased risk of adverse events during the waiting period.52 A recent metaanalysis of observational studies calculated that a waiting period of 3 months for surgical myocardial revascularization may be associated with the risk of 1 death among 80 patients.53 Table 3 shows the preferred timing of revascularization depending on the clinical presentation
and the extent and localization of CAD.54
[Beware of inappropriate Ad Hoc PCI]
Ad hoc PCI is defined as a therapeutic intervention performed
within the same procedure as the diagnostic coronary angiography. Ad hoc PCI is convenient, often cost-effective and safe, and is associated with fewer access site complications and lower radiation exposure.55,56 However, in the USA, up to 30% of patients undergoing ad hoc PCI are potential candidates for CABG.56 This number may be lower in Europe.45 Although it is not advisable for ad hoc PCI to represent the default approach for complex SCAD, it may be justified if a full diagnostic work-up, including functional testing, is available and the patient is adequately informed on both percutaneous and surgical
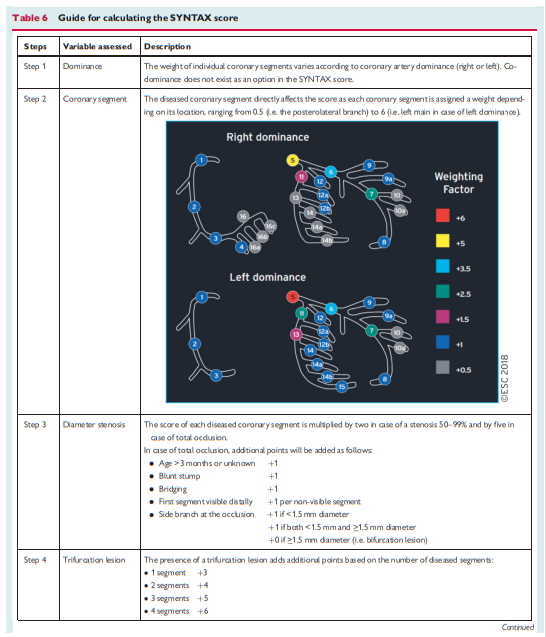
myocardial revascularization options (see section 4.1). Institutional protocols developed by the Heart Team in accordance with current Guidelines should define specific anatomical criteria and clinical subsets that may be—or should not be—treated ad hoc. Stable patients with complex CAD, as reflected by a high SYNTAX score [See Resource (4) below in Resources], should in general be discussed by the Heart Team and not be treated ad hoc.5 Revascularization for stable coronary artery disease
So decision making in Stable Coronary Artery Disease is covered in the 2018 Guidelines from pp 8 – 24.
The 2018 Guidelines suggest careful review of 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology [Resource 3 below]
A quick way to review the 2013 Guidelines is by simply reviewing all of the figures and tables in the article. See the list of both in the Table Of Contents.
Resources
(1) 2018 ESC/EACTS Guidelines on myocardial revascularization [PubMed Citation – Abstract Not Yet Available] [Full Text HTML] [Full Text PDF]. Eur Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy394. [Epub ahead of print].
(2) Considerations for the choice between coronary artery bypass grafting and percutaneous coronary intervention as revascularization strategies in major categories of patients with stable multivessel coronary artery disease: an accompanying article of the task force of the 2018 ESC/EACTS guidelines on myocardial revascularization] [PubMed Citation – Abstract Not Yet Available] [Full text of the article is available to nonsubscribers only by Purchase]. Eur Heart J. 2018 Aug 25. doi: 10.1093/eurheartj/ehy532. [Epub ahead of print].
(3) 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology [PubMed Abstract] [Full Text HTML]. Eur Heart J. 2013 Oct;34(38):2949-3003. doi: 10.1093/eurheartj/eht296. Epub 2013 Aug 30.
Erratum in
- Eur Heart J. 2014 Sep 1;35(33):2260-1.
(4) Calculating the SYNTAX Score
(5) ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease [PubMed Abstract] [Full Text HTML] [Full Text PDF].
This article has a correction. Please see:
And here are the corrections:
J Am Coll Cardiol 2017;69:2212–41.
To more clearly reflect the relationship between iFR (instantaneous wave-free ratio) and FFR (fractional flow reserve), the following changes were made to the document after its initial publication.
1. Page 2218, the following sentence has been added to the end of the final paragraph of Section 2. “Methods”: “Other physiologic measurements using the ratio of distal coronary to aortic pressure without hyperemia exist, and correlate with FFR, but are not as well-validated and lack the clinical outcomes data existing for FFR.”
2. Page 2218, Section 3. “Assumptions”, “General Assumptions”. Assumption 1 previously comprised the following sentence: “When available, each clinical scenario includes the patient’s clinical status/symptom complex, ischemic burden as determined by noninvasive functional testing, burden of coronary atherosclerosis as determined by angiography, and additional invasive testing evaluations by invasive physiology (e.g., FFR, instantaneous wave-free ratio) or intravascular imaging.” The parenthetical text in that sentence has been amended to read “(e.g., FFR or other physiological pressure measurements not requiring hyperemia)”.
3. Page 2218, Section 3. “Assumptions”, “General Assumptions”. The following sentence has been added to the end of assumption 6: “FFR is the reference standard for invasively assessing the physiological significance of a coronary artery stenosis before PCI. Newer physiological measurements that do not require hyperemia measure the ratio of distal coronary pressure to aortic pressure during the whole cardiac cycle or the wave-free portion of the cycle. Both indices have similar diagnostic concordance with FFR but have different normal values and have not been as well-studied as FFR. Substitution of one of the newer physiological measurements for FFR may be considered provided the appropriate reference values are used.”
4. Page 2224, Table 1.1. “One-Vessel Disease”. The footnote previously read, “*iFR measurements with appropriate normal range may be substituted for FFR.” The footnote now reads, “*Substitution of a newer coronary pressure ratio that does not require hyperemia for FFR may be considered provided the appropriate reference values are used.” The following item has been removed from the list of abbreviations beneath the table: “iFR, instant wave-free ratio”. The same corrections have been made to Table 1.2.” “Two-Vessel Disease”, Table 2.1. “IMA to LAD Patent and Without Significant Stenoses”, and Table 2.2. “IMA to LAD Not Patent”.
5. Page 2228, Section 7. “Discussion”. The eighth sentence in the second paragraph previously read, “Fourth, the scenarios expand the use of intracoronary physiological testing, mainly with FFR.” That sentence now reads, “Fourth, the scenarios expand the use of intracoronary physiological testing, which should be performed primarily with FFR as it is a well-validated measurement and is associated with clinical outcomes following PCI.”
- 2018 American College of Cardiology Foundation