Today I link to and review A Framework for Evaluation of the Higher-Risk Infant After a Brief Resolved Unexplained Event [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Pediatrics. 2019 Aug;144(2):e20184101. doi: 10.1542/peds.2018-4101. Links to all similar articles in PubMed.
All that follows is from the above resource.
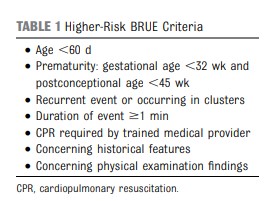
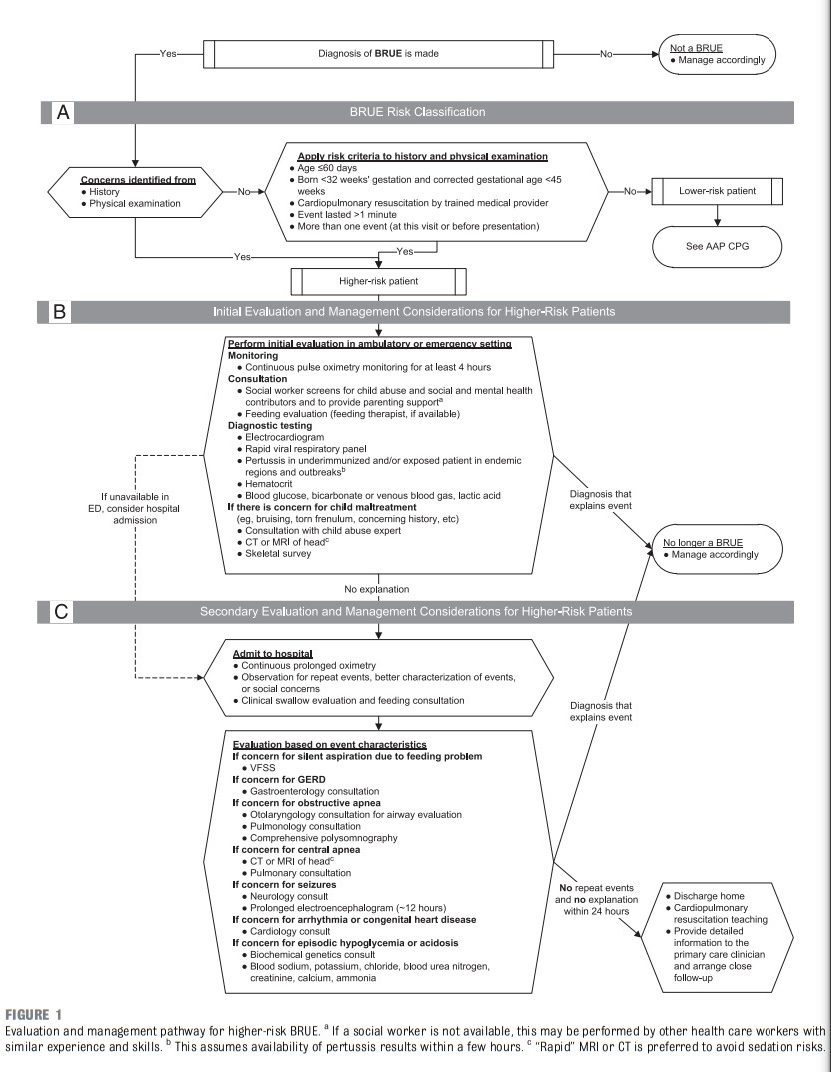
The AAP CPG [on Brief Resolved Unexplained Events (BRUE)] recommendations apply only to infants defined as “lower risk.” Lower-risk infants are defined as (1) age >60 days, (2) gestational age ≥32 weeks and postconceptional age ≥45 weeks, (3) no cardiopulmonary resuscitation required by trained medical provider, (4) BRUE duration <1 minute, and (5) first BRUE (no repeat events, regardless of time interval) as well as having no concerning historical features (eg, sudden death in a relative) and no concerning physical examination findings (eg, unexplained bruising).1 There are currently no recommendations for infants who do not meet these criteria and are considered higher risk, despite this situation being common and challenging to manage. In this article, we propose a framework to guide the evaluation and management of infants meeting higher-risk criteria (Table 1). Because of a paucity of research on higher-risk infants, we do not provide recommendations in the format of a CPG. Rather, we offer an evidence-informed framework to guide initial medical decision-making, ongoing research, and improvements in managing this population.
INITIAL EVALUATION AND MANAGEMENT
For higher-risk infants, initial tier evaluations (Fig 1B) may identify problems sensitive to delays in
diagnosis and treatment. Hospitalization may not be needed
for many patients at this stage, particularly if close follow-up with a primary care clinician can be arranged and the services are available either in the emergency department (ED) or outpatient setting.These may include the following:
• continuous pulse oximetry monitoring for at least 4 hours;
• consultation with social worker (or other health care worker with similar experience and skills);
• bedside feeding evaluation (by a feeding therapist, if available);
• electrocardiogram to be read by a pediatric cardiologist or electrophysiologist;
• rapid viral respiratory panel testing;
• rapid pertussis polymerase chain reaction in endemic areas, during regional outbreaks, or in underimmunized patients;
• hematocrit;
• blood glucose, bicarbonate or venous blood gas, and lactate; and
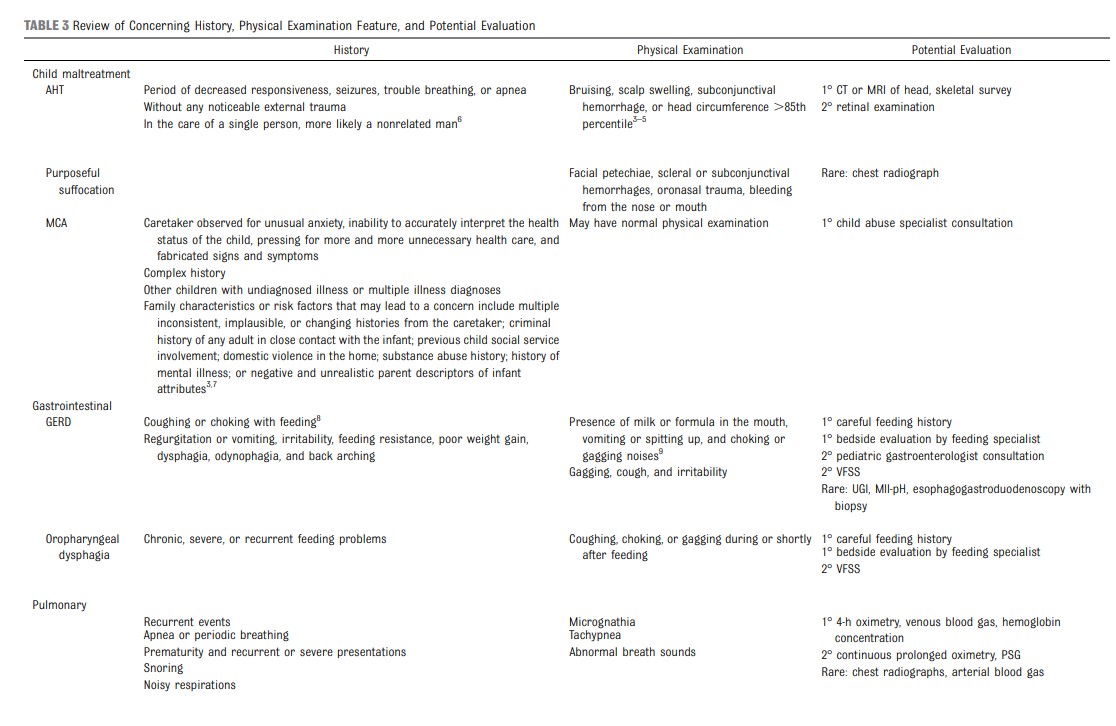
• if concerned for child maltreatment, consultation with
a child abuse expert, head imaging with computed tomography (CT) or MRI, and skeletal survey. (Note that evidence supporting these recommendations is reviewed in
subsequent sections.)SECONDARY EVALUATION AND MANAGEMENT
If no explanation is identified through the initial tier of evaluations, secondary tier evaluations may be
tailored to specific individual concerns remaining from previously identified characteristics, findings, and evaluations or clinician and/or caregiver concerns in the history and
physical examination. Depending on family preference and other circumstances, it is reasonable to perform the secondary tier of evaluations after discharge (Fig 1C).It is also reasonable to admit the infant to the hospital for a period of observation, continuous prolonged oximetry, and clinical swallow evaluation or feeding specialist consultation to better assess recurrent events and complete evaluations that could not be otherwise arranged in the ambulatory setting.
Secondary tier evaluations can include any of the following consultations:
• gastroenterology;
• otolaryngology;
• pulmonary or sleep expert;
• child abuse expert;
• neurology;
• cardiology; and
• biochemical genetics.Secondary tier evaluations that may
be considered in combination with
specialty consultation are as follows:• videofluoroscopic swallowing
study (VFSS) for “silent” oropharyngeal dysphagia not seen in bedside evaluation;
• continuous prolonged oximetry to characterize recurring events;
• comprehensive polysomnography (PSG) to characterize and quantify central versus obstructive apnea;
• prolonged (≥ 12–24 hours) EEG; and
• blood sodium, potassium, chloride, blood urea nitrogen, creatinine, calcium, and ammonia for metabolic disturbance.