In addition to this post please see Pertussis In Infants and Children – What Can We Do To Prevent Tragedy? Posted on June 23, 2014 and Do You Or Your Child Have Whooping Cough? Here’s How to Tell Posted on February 10, 2012
Everything in this post is from Pink Book’s Chapter on Pertussis pp 261-278 from Epidemiology & Prevention of Vaccine-Preventable Diseases textbook 13th Ed. (2015) What follows are some excerpts from that chapter:
Pertussis remains a major health problem among children
in developing countries, with 195,000 deaths resulting from
the disease in 2008 (World Health Organization estimate).The first stage, the catarrhal stage, is characterized by the
insidious onset of coryza (runny nose), sneezing, low-grade
fever, and a mild, occasional cough, similar to the common
cold. The cough gradually becomes more severe, and after
1–2 weeks, the second, or paroxysmal stage, begins. Fever is
generally minimal throughout the course of the illness.It is during the paroxysmal stage that the diagnosis of
pertussis is usually suspected. Characteristically, the patient
has bursts, or paroxysms, of numerous, rapid coughs,
apparently due to difficulty expelling thick mucus from the
tracheobronchial tree. At the end of the paroxysm, a long
inspiratory effort is usually accompanied by a characteristic
high-pitched whoop. During such an attack, the patient may
become cyanotic (turn blue). Children and young infants,
especially, appear very ill and distressed. Vomiting and
exhaustion commonly follow the episode. The person does
not appear to be ill between attacks.Paroxysmal attacks occur more frequently at night, with
an average of 15 attacks per 24 hours. During the first 1
or 2 weeks of this stage, the attacks increase in frequency,
remain at the same level for 2 to 3 weeks, and then gradually
decrease. The paroxysmal stage usually lasts 1 to 6 weeks
but may persist for up to 10 weeks. Infants younger than 6
months of age may not have the strength to have a whoop,
but they do have paroxysms of coughing.In the convalescent stage, recovery is gradual. The cough
becomes less paroxysmal and disappears in 2 to 3 weeks.
However, paroxysms often recur with subsequent respiratory
infections for many months after the onset of pertussis.Adolescents, adults and children partially protected by the
vaccine may become infected with B. pertussis but may have
milder disease than infants and young children. Pertussis
infection in these persons may be asymptomatic, or present
as illness ranging from a mild cough illness to classic
pertussis with persistent cough (i.e., lasting more than
7 days). Inspiratory whoop is not common.Even though the disease may be milder in older persons, those who are infected may transmit the disease to other susceptible persons, including unimmunized or incompletely immunized infants. Older persons are often found to have the first case in a household with multiple pertussis cases, and are often the source of infection for children. [Emphasis Added]
Pertussis
● Acute infectious disease caused by Bordetella pertussis
● Outbreaks first described in 16th century
● Bordetella pertussis isolated in 1906
● Estimated 195,000 deaths worldwide in 2008
Complications
The most common complication, and the cause of most
pertussis-related deaths, is secondary bacterial pneumonia.
Young infants are at highest risk for acquiring pertussis-associated complications. Data from 1997–2000 indicate
that pneumonia occurred in 5.2% of all reported pertussis
cases, and among 11.8% of infants younger than 6 months
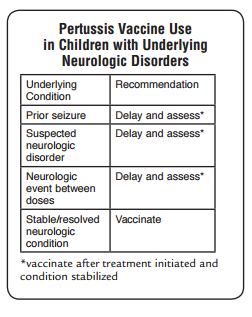
of age.Neurologic complications such as seizures and encephalopathy
(a diffuse disorder of the brain) may occur as a result
of hypoxia (reduction of oxygen supply) from coughing, or
possibly from toxin. Neurologic complications of pertussis
are more common among infants. Other less serious
complications of pertussis include otitis media, anorexia,
and dehydration. Complications resulting from pressure
effects of severe paroxysms include pneumothorax, epistaxis,
subdural hematomas, hernias, and rectal prolapse.In 2008 through 2011 a total of 72 deaths from pertussis were reported to CDC. Children 3 months of age or younger accounted for 60 (83%) of these deaths. During 2008-2011, the annual mean of pertussis cases in infants was 3,132 (range 2,230 – 4,298), the mean of hospitalizations was 1,158 (range 687-1,459) and the mean of deaths was 16 (range 11-25). [Emphasis Added]
Bordetella pertussis
● Fastidious gram-negative bacteria
● Antigenic and biologically active components:
■ pertussis toxin (PT)
■ filamentous hemagglutinin
(FHA)
■ agglutinogens
■ adenylate cyclase
■ pertactin
■ tracheal cytotoxin
Pertussis Pathogenesis
● Primarily a toxin-mediated disease
● Bacteria attach to cilia of respiratory epithelial cells
● Inflammation occurs which interferes with clearance of pulmonary secretions
● Pertussis antigens allow evasion of host defenses(lymphocytosis promoted but
impaired chemotaxis)
Pertussis Clinical Features
● Incubation period 7-10 days
(range 4-21 days)
● Insidious onset, similar to the common cold with nonspecific cough
● Fever usually minimal throughout course of illness
● Catarrhal stage
■ 1-2 weeks
● Paroxysmal cough stage
■ 1-6 weeks
● Convalescence
■ weeks to months
Pertussis Among Children, Adolescents and Adults
● Disease often milder than in infants and young children
● Infection may be asymptomatic, or may present as classic pertussis
● Persons with mild disease may transmit the infection
● Older persons often source of infection for children
Pertussis Complications in Children
● Secondary bacterial pneumonia – most common
● Neurologic complications – seizures, encephalopathy more common among infants
● Otitis media
● Anorexia
● Dehydration
● Pneumothorax
● Epistaxis
● Subdural hematomas
● Hernias
● Rectal prolapse
Pertussis Complications in Adolescents and Adults
● Difficulty sleeping
● Urinary incontinence
● Pneumonia
● Rib fracture
Pertussis Laboratory Diagnosis
● Culture – gold standard
● Polymerase Chain Reaction (PCR)
■ can confirm pertussis in an outbreak
■ highly sensitive
■ high false-positive rate
● Serology
■ can confirm illness late in the course of infection
■ many tests have unproven or unknown clinical accuracy
● Direct fluorescent
antibody test
■ low sensitivity
■ variable specificity
■ should not be used for
laboratory confirmation
Pertussis Epidemiology
● Reservoir
■ Human Adolescents
and adults
● Transmission
■ Respiratory droplets
● Communicability
■ Maximum in catarrhal stage
■ Secondary attack rate up
to 80%
Whole-Cell Pertussis Vaccine
● Developed in 1930s and used widely in clinical practice
through mid-1940s
● DTP – 70%-90% effective after 4 doses
● Little to no protection after 5-10 years
● Local adverse reactions common
Pertussis-containing Vaccines
● DTaP (pediatric)
● approved for children 6 weeks through 6 years (to age 7
years)
● Tdap (adolescent and adult)
● approved for persons 10 years and older (Boostrix) and 10 through 64 years (Adacel)
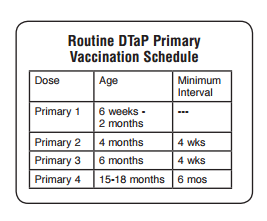
DTaP Fourth Dose
● Recommended at 15-18 months*
● May be given at 12 months
of age if:
■ 6 months since DTaP3, and
■ unlikely to return at 15-18 months
*15-20 months for Daptacel
School Entry (Fifth) Dose
● Fifth dose recommended when 4th dose given before age 4 years
● All DTaP vaccines are licensed for 5th dose after DTaP series
Interchangeability of Different Brands of DTaP Vaccine
● Series should be completed with same brand of vaccine if possible
● Limited data suggest that “mix and match” DTaP schedules do not adversely affect safety and immunogenicity
● Use different brand of DTaPif necessary
Tdap Vaccines
● Boostrix (GlaxoSmithKline)
■ approved for persons 10 years of age and older
● Adacel (sanofi pasteur)
■ approved for persons 10 through 64 years of age
Tdap Recommendations
● A single dose of Tdap is recommended for
■ adolescents 11 through 18 years of age
■ adults 19 and older
■ children 7-10 years of age who are not fully vaccinated against pertussis*
* “Not fully vaccinated” against pertussis is defined as having received fewer than 4 doses of DTaP, or having received 4 doses of DTaP but the last dose was prior to age 4 years. See MMWR 2011;60(No.1):13-5.
Tdap Recommendations for Pregnant Women
● Providers of prenatal care should implement a Tdapvaccination program for pregnant women who previously have not received Tdap
● Administer Tdap in each pregnancy, preferably at 27 through 36 weeks gestation
● If not administered during pregnancy, Tdap should be administered immediately postpartum, for women not previously vaccinated with Tdap
MMWR 2013;62(No. 7):131-5
Tdap Vaccine and Healthcare Personnel
● Healthcare personnel should receive a single dose of Tdap as soon as feasible*
● Priority should be given to vaccination of healthcare personnel who have direct contact with infants 12 months of age and younger
*if they have not previously received Tdap. MMWR 2006;55(RR-17):1-37
Tdap For Persons Without A History of DTP or DTaP
● All adolescents and adults should have documentation
of having received a series of DTaP, DTP, DT, or Td
● Persons without
documentation should receive a series of 3 vaccinations
● One dose should be Tdap, preferably the first
Pediarix
● DTaP – Hep B – IPV combination
● Minimum age 6 weeks
● Approved for 3 doses at 2, 4 and 6 months
● Not approved for 4th or 5th booster dose of DTaP or IPV series
● Licensed for children 6 weeks through 6 years of age
● May be used interchangeably with other pertussis containing vaccines if necessary
● Can be given at 2, 4, and 6 months in infants who received a birth dose of
hepatitis B vaccine (total of 4 doses)
● May be used in infants whose mothers are HBsAg positive or
status is not known*
*Off-label ACIP recommendation https://www.cdc.gov/vaccines/programs/
vfc/downloads/resolutions/1003-hepb.pdf
Pentacel Vaccine
● Contains lyophilized Hib (ActHIB) vaccine that is reconstituted with a liquid DTaP-IPV solution
● Approved for doses 1 through 4 among children 6 weeks through 4 years of age
● The DTaP-IPV solution should not be used separately (i.e., only use to reconstitute the Hib component)
Kinrix
Kinrix is a combination vaccine that contains DTaP and
inactivated poliovirus vaccine (IPV) that is produced by
GlaxoSmithKline. It was approved by the FDA in 2008. Kinrix
is licensed only for the fifth dose of DTaP and fourth dose
of IPV in children 4 through 6 years of age whose previous
DTaP vaccine doses have been with Infanrix and/or Pediarix
for the first three doses and Infanrix for the fourth dose.
However, if Kinrix is administered to children who received
another brand of DTaP for prior DTaP doses the Kinrix dose
does not need to be repeated.
DTaP Contraindications
● Severe allergic reaction to vaccine component or following a prior dose
● Encephalopathy not due to another identifiable cause occurring within 7 days after vaccination
DTaP Precautions*
● Moderate or severe acute illness
● Temperature 105°F (40.5°C) or higher within 48 hours with no other identifiable cause
● Collapse or shock-like state (hypotonic-hyporesponsive episode) within 48 hours
● Persistent, inconsolable crying lasting 3 hours or longer,occurring within 48 hours
● Convulsions with or without fever occurring within 3 days
*may consider use in outbreaks
Tdap Contraindications
● Severe allergic reaction to vaccine component or following a prior dose
● Encephalopathy not due to another identifiable causeoccurring within 7 days after vaccination with a pertussis containing vaccine
DTaP Adverse Reactions
● Local reactions (pain, redness, swelling)
■ 20%-40%
● Temp of 101°F
■ 3%-5% or higher
● More severe adverse reactions
■ not common
● Local reactions more common following 4th and 5th doses
Adverse Reactions Following the 4th and 5th DTaP Dose
● Local adverse reactions and fever increased with 4th and
5th doses of DTaP
● Reports of swelling of entire limb
● Extensive swelling after 4th dose NOT a contraindication to 5th dose
Tdap Adverse Reactions
● Local reactions (pain, redness, swelling)
■ 21%-66%
● Temp of 100.4°F or higher
■ 1.4%
● Adverse reactions occur at approximately the same rate as Td alone (without acellular pertussis vaccine)