The tables in my post 2017 ISDA Guidelines For Infectious Diarrhea In Adults And Children were mostly unreadable [copies from the PDF of the Guidelines]. Therefore, I redid the post today making the tables readable by using the HTML tables excerpted from the article.
The Executive Summary of Resource (1) [HTML] runs from p e45 to e55 of the PDF. You can also easily read the Executive Summary in the HTML. Please read the executive summary first [note to myself when reviewing again].
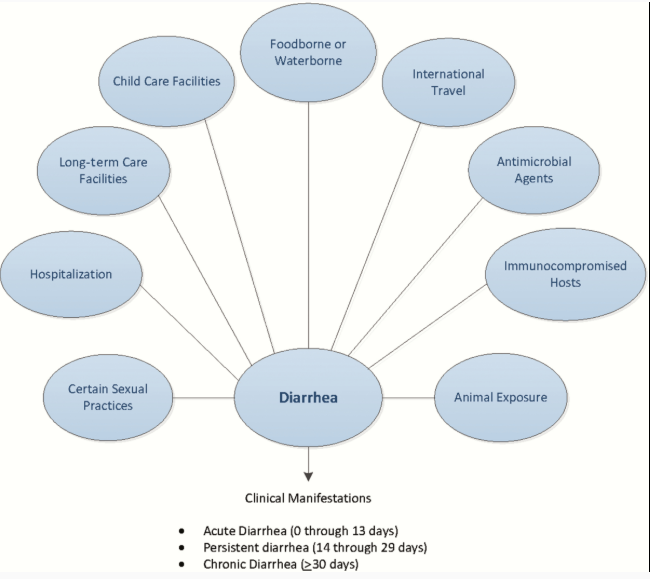
Only after reading the executive summary should you go ahead and review the Figures and Tables I’ve included in my study notes will give you an adequate quick review [Again just a note to myself].
But the reading the full text and the Additional Resources after the post will give you the complete picture of Infectious Diarrhea.
The following Figures And Tables are from Resource (1) below, The 2017 IDSA Infectious Diarrhea Guidelines:
Table 1.Modes of Acquisition of Enteric Organisms and Sources of Guidelines
Mode Title URL Author/Issuing Agency International travel Expert Review of the Evidence Base for Prevention of Travelers’ Diarrhea https://www.ncbi.nlm.nih.gov/pubmed/19538575 DuPont et al [113] Medical Considerations Before International Travel https://www.ncbi.nlm.nih.gov/pubmed/27468061 Freedman et al [207] The Yellow Book https://wwwnc.cdc.gov/travel/page/ yellowbook-home-2014 CDC Travelers Health https://wwwnc.cdc.gov/travel CDC Immunocompromised hosts Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents https://aidsinfo.nih.gov/contentfiles/lvguidelines/ adult_oi.pdf CDC/NIH/HIVMA/IDSA Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-Infected Children https://aidsinfo.nih.gov/contentfiles/lvguidelines/ oi_guidelines_pediatrics.pdf CDC/NIH/HIVMA/IDSA Foodborne and waterborne Surveillance for Foodborne Disease Outbreaks—United States, 2009–2010 https://www.cdc.gov/mmwr/preview/mmwrhtml/ mm6203a1.htm?s_cid=mm6203a1_w CDC Food Safety https://www.cdc.gov/foodsafety/ CDC Healthy Water https://wwwnc.cdc.gov/healthywater CDC Antimicrobial-associated (C. difficile) Clinical Practice Guidelines for Clostridium difficileInfection in Adults and Children 2017 Update (in press) https://www.jstor.org/stable/10.1086/651706 IDSA/SHEA 2010 Clinical Practice Guidelines for Clostridium difficileInfection in Adults https://www.idsociety.org/ Organ_System/#Clostridiumdifficile IDSA/SHEA Healthcare-associated Healthcare-Associated Infections https://www.cdc.gov/hai/ CDC Child care settings Caring for Our Children: National Health and Safety Performance Standards; Guidelines for Early Care and Education Programs https://nrckids.org. AAP, APHA, NRC Recommendations for Care of Children in Special Circumstances—Children in Out- of-Home Child Care (pp 132–51) https://redbook.solutions.aap.org/redbook.aspx AAP Managing Infectious Diseases in Child Care and Schools https://ebooks.aappublications.org/content/managing-infectious-diseases-in-child-care-and-schools- 3rd-edition AAP Long-term care settings Nursing Homes and Assisted Living (Long- term Care Facilities) https://www.cdc.gov/longtermcare/ CDC Infection Prevention and Control in the Long-term Care Facility https://www.shea-online.org/assets/files/position-papers/ic-ltcf97.pdf SHEA/APIC Zoonoses Compendium of Measures to Prevent Disease Associated With Animals in Public Settings https://www.cdc.gov/mmwr/preview/mmwrhtml/ rr6004a1.htm?s_cid=rr6004a1_w CDC Exposure to Nontraditional Pets at Home and to Animals in Public Settings: Risks to Children https://pediatrics.aappublications.org/ content/122/4/876 Pickering et al [51] Review of Institute of Medicine and National Research Council Recommendations for One Health Initiative https://wwwnc.cdc.gov/eid/article/19/12/12-1659_ article.htm Rubin et al [208] Abbreviations: AAP, American Academy of Pediatrics; APHA, American Public Health Association; APIC, Association for Professionals in Infection Control and Epidemiology; CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; HIVMA, HIV Medicine Association; IDSA, Infectious Diseases Society of America; NIH, National Institutes of Health; NRC, National Resource Center for Health and Safety in Child Care and Early Education; SHEA, Society for Healthcare Epidemiology of America.
Table 2.Exposure or Condition Associated With Pathogens Causing Diarrhea
Exposure or Condition Pathogen(s) Foodborne Foodborne outbreaks in hotels, cruise ships, resorts, restaurants, catered events Norovirus, nontyphoidal Salmonella, Clostridium perfringens, Bacillus cereus, Staphylococcus aureus, Campylobacter spp, ETEC, STEC, Listeria, Shigella, Cyclospora cayetanensis, Cryptosporidium spp Consumption of unpasteurized milk or dairy products Salmonella, Campylobacter, Yersinia enterocolitica, S. aureus toxin, Cryptosporidium, and STEC. Listeria is infrequently associated with diarrhea, Brucella (goat milk cheese), Mycobacterium bovis, Coxiella burnetii Consumption of raw or undercooked meat or poultry STEC (beef), C. perfringens (beef, poultry), Salmonella (poultry), Campylobacter (poultry), Yersinia (pork, chitterlings), S. aureus(poultry), and Trichinella spp (pork, wild game meat) Consumption of fruits or unpasteurized fruit juices, vegetables, leafy greens, and sprouts STEC, nontyphoidal Salmonella, Cyclospora, Cryptosporidium, norovirus, hepatitis A, and Listeria monocytogenes Consumption of undercooked eggs Salmonella, Shigella (egg salad) Consumption of raw shellfish Vibrio species, norovirus, hepatitis A, Plesiomonas Exposure or contact Swimming in or drinking untreated fresh water Campylobacter, Cryptosporidium, Giardia, Shigella, Salmonella, STEC, Plesiomonas shigelloides Swimming in recreational water facility with treated water Cryptosporidium and other potentially waterborne pathogens when disinfectant concentrations are inadequately maintained Healthcare, long-term care, prison exposure, or employment Norovirus, Clostridium difficile, Shigella, Cryptosporidium, Giardia, STEC, rotavirus Child care center attendance or employment Rotavirus, Cryptosporidium, Giardia, Shigella, STEC Recent antimicrobial therapy C. difficile, multidrug-resistant Salmonella Travel to resource-challenged countries Escherichia coli (enteroaggregative, enterotoxigenic, enteroinvasive), Shigella, Typhi and nontyphoidal Salmonella, Campylobacter, Vibrio cholerae, Entamoeba histolytica, Giardia, Blastocystis, Cyclospora, Cystoisospora, Cryptosporidium Exposure to house pets with diarrhea Campylobacter, Yersinia Exposure to pig feces in certain parts of the world Balantidium coli Contact with young poultry or reptiles Nontyphoidal Salmonella Visiting a farm or petting zoo STEC, Cryptosporidium, Campylobacter Exposure or condition Age group Rotavirus (6–18 months of age), nontyphoidal Salmonella (infants from birth to 3 months of age and adults >50 years with a history of atherosclerosis), Shigella (1–7 years of age), Campylobacter (young adults) Underlying immunocompromising condition Nontyphoidal Salmonella, Cryptosporidium, Campylobacter, Shigella, Yersinia Hemochromatosis or hemoglobinopathy Y. enterocolitica, Salmonella AIDS, immunosuppressive therapies Cryptosporidium, Cyclospora, Cystoisospora, microsporidia, Mycobacterium avium–intercellulare complex, cytomegalovirus Anal-genital, oral-anal, or digital-anal contact Shigella, Salmonella, Campylobacter, E. histolytica, Giardia lamblia, Cryptosporidium as well as sexually transmitted infections Abbreviations: ETEC, enterotoxigenic Escherichia coli; STEC, Shiga toxin–producing Escherichia coli.
Table 3.Clinical Presentations Suggestive of Infectious Diarrhea Etiologies
Finding Likely Pathogens Persistent or chronic diarrhea Cryptosporidium spp, Giardia lamblia, Cyclospora cayetanensis, Cystoisospora belli, and Entamoeba histolytica Visible blood in stool STEC, Shigella, Salmonella, Campylobacter, Entamoeba histolytica, noncholera Vibrio species, Yersinia, Balantidium coli, Plesiomonas Fever Not highly discriminatory—viral, bacterial, and parasitic infections can cause fever. In general, higher temperatures are suggestive of bacterial etiology or E. histolytica. Patients infected with STEC usually are not febrile at time of presentation Abdominal pain STEC, Salmonella, Shigella, Campylobacter, Yersinia, noncholera Vibrio species, Clostridium difficile Severe abdominal pain, often grossly bloody stools (occasionally nonbloody), and minimal or no fever STEC, Salmonella, Shigella, Campylobacter, and Yersinia enterocolitica Persistent abdominal pain and fever Y. enterocolitica and Y. pseudotuberculosis; may mimic appendicitis Nausea and vomiting lasting ≤24 hours Ingestion of Staphylococcus aureus enterotoxin or Bacillus cereus(short-incubation emetic syndrome) Diarrhea and abdominal cramping lasting 1–2 days Ingestion of Clostridium perfringens or B. cereus (long-incubation emetic syndrome) Vomiting and nonbloody diarrhea lasting 2–3 days or less Norovirus (low-grade fever usually present during the first 24 hours in 40% if infections) Chronic watery diarrhea, often lasting a year or more Brainerd diarrhea (etiologic agent has not been identified); postinfectious irritable bowel syndrome Abbreviation: STEC, Shiga toxin–producing Escherichia coli.
Table 4.Postinfectious Manifestations Associated With Enteric Pathogens
Manifestation Organism(s) Erythema nodosum Yersinia, Campylobacter, Salmonella, Shigella Glomerulonephritis Shigella, Campylobacter, Yersinia Guillain-Barré syndrome Campylobacter Hemolytic anemia Campylobacter, Yersinia Hemolytic uremic syndrome STEC, Shigella dysenteriae serotype 1 Immunoglobulin A nephropathy Campylobacter Reactive arthritisa Salmonella, Shigella, Campylobacter, Yersinia, rarely Giardia, and Cyclospora cayetanensis Postinfectious irritable bowel syndrome Campylobacter, Salmonella, Shigella, STEC, Giardia Meningitis Listeria, Salmonella (infants ≤3 months of age are at high risk) Intestinal perforation Salmonella including Salmonella Typhi, Shigella, Campylobacter, Yersinia, Entamoeba histolytica Ekiri syndrome (lethal, toxic encephalopathy) and/or seizure Shigella Aortitis, osteomyelitis, extravascular deep tissue focus Salmonella, Yersinia Abbreviation: STEC, Shiga toxin–producing Escherichia coli.
aIncludes Reiter syndrome.
Table 5.Laboratory Diagnostics for Organisms Associated With Infectious Diarrhea
Etiologic Agent Diagnostic Procedures Optimal Specimen Clostridium difficile NAAT Stool GDH antigen with or without toxin detection followed by cytotoxin or Clostridium difficile toxin or toxigenic C. difficilestrain Salmonella enterica, Shigella spp, Campylobacterspp Routine stool enteric pathogen culturea or NAAT Stool Salmonella enterica serovars Typhi and Paratyphi (enteric fever) Routine culture Stool, blood, bone marrow, and duodenal fluid Shiga toxin–producing Escherichia coli Culture for E. coliO157:H7b
and Shiga toxin immunoassay or NAAT for Shiga toxin genesStool Yersinia spp, Plesiomonas spp, Edwardsiella tarda, Staphylococcus aureus, E. coli (enterotoxigenic, enteroinvasive, enteropathogenic, enteroaggregative) Specialized stool culture or molecular assaysc or NAAT Stool Clostridium perfringens Specialized procedure for toxin detectiond Stool Bacillus cereus, S. aureus Specialized procedure for toxin detectiond Food Clostridium botulinum Mouse lethality assay (performed at a state public health laboratory, or CDC) e,f,g Serum, stool, gastric contents, vomitus Entamoebahistolytica; Blastocystis hominih;
Dientamoeba fragilish; Balantidium coli; Giardia lamblia; nematodes (generally not associated with diarrhea) including Ascaris lumbricoides, Strongyloides stercoralisi, Trichuris trichiura, hookworms; cestodes (tapeworms); trematodes (flukes)Ova and parasite examination including permanent stained smeari or NAAT Stool
Duodenal fluid for Giardia and StrongyloidesE. histolytica E. histolytica species-specific
immunoassay or NAATStool G. lambliaj EIA or NAAT Stool Cryptosporidium spp [121]j Direct fluorescent immunoassay, EIA, or NAAT Stool Cyclospora cayetanensis, Cystoisospora bellik Modified acid-fast stainkperformed on
concentrated specimen, ultraviolet fluorescence microscopy, or NAATStool Microsporidia (now classified as a fungus) Modified trichrome stainkperformed on
concentrated specimenStool Histologic examination with electron microscopic confirmation Small bowel biopsy Calicivirus (norovirus, sapovirus)k; enteric adenovirus; enterovirus/ parechovirusk; rotavirus NAAT Stool Rotavirus, enteric adenovirus EIA Stool Enteric adenovirusl; enterovirus/parechovirus Viral culture Stool Cytomegalovirus Histopathological examination Biopsy Cytomegalovirus culture Biopsy The field of rapid diagnostic testing is rapidly expanding. We expect that additional diagnostic assays will become available following publication of these guidelines, specifically panel-based molecular diagnostics, including NAAT. Contact the laboratory for instructions regarding container, temperature, and transport guidelines to optimize results.
Abbreviations: CDC, Centers for Disease Control and Prevention; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification test.
aRoutine stool culture in most laboratories is designed to detect Salmonella spp, Shigella spp, Campylobacter spp, and E. coli O157 or Shiga toxin–producing E. coli, but this should be confirmed with the testing laboratory.
bIt is recommended that laboratories routinely process all stool specimens submitted for bacterial culture for the presence of Shiga toxin–producing strains of E. coli including O157:H7. However, in some laboratories, O157:H7 testing is performed only by specific request.
cSpecialized cultures or molecular assays may be required to detect these organisms in stool specimens. The laboratory should be notified whenever there is a suspicion of infection due to one of these pathogens.
dBacillus cereus, Clostridium perfringens, and Staphylococcus aureus are associated with diarrheal syndromes that are toxin mediated. An etiologic diagnosis is made by demonstration of toxin in stool for C. perfringens and demonstration of toxin in food for B. cereus and S. aureus.
eToxin assays are either performed in public health laboratories or referred to laboratories specializing in such assays.
fTesting for Clostridium botulinum toxin is either performed in public health laboratories or referred to laboratories specializing in such testing. The toxin is lethal and special precautions are required for handling. Class A bioterrorism agent and rapid sentinel laboratory reporting schemes must be followed. Immediate notification of a suspected infection to the state health department is mandated.
gImplicated food materials may also be examined for C. botulinum toxin, but most hospital laboratories are not equipped for food analysis.
hThe pathogenicity of Blastocystis hominis and Dientamoeba fragilis remains controversial. In the absence of other pathogens, they may be clinically relevant if symptoms persist. Reporting semiquantitative results (rare, few, many) may help determine significance and is a College of American Pathologists accreditation requirement for participating laboratories.
iDetection of Strongyloides in stool may require the use of Baermann technique or agar plate culture.
jCryptosporidium and Giardia lamblia testing is often offered and performed together as the primary parasitology examination. Further studies should follow if the epidemiologic setting or clinical manifestations suggest parasitic disease.
kThese stains may not be routinely available.
lEnteric adenoviruses may not be recovered in routine viral culture.
Table 6.Recommended Antimicrobial Agents by Pathogen
Indication First Choice Alternative Comments/Considerations Bacteriaa Campylobacter Azithromycin Ciprofloxacin Clostridium difficile Oral vancomycin Fidaxomicin Fidaxomicin not currently recommended for people <18 years of age. Metronidazole is still acceptable treatment for nonsevere CDI in children and as a second-line agent for adults with nonsevere CDI (eg, who cannot obtain vancomycin or fidaxomicin at a reasonable cost). Nontyphoidal Salmonella entericab Usually not indicated for uncomplicated infection NA Antimicrobial therapy should be considered for groups at increased risk for invasive infection: neonates (up to 3 months old), persons >50 years old with suspected atherosclerosis, persons with immunosuppression, cardiac disease (valvular or endovascular), or significant joint disease. If susceptible, treatment with ceftriaxone, ciprofloxacin, TMP-SMX, or amoxicillin. Salmonella enterica Typhi or Paratyphib Ceftriaxone or ciprofloxacin Ampicillin or TMP-SMX or azithromycin Shigellaa Azithromycincor ciprofloxacina, or ceftriaxone TMP-SMX or ampicillin if susceptible Clinicians treating people with shigellosis for whom antibiotic treatment is indicated should avoid prescribing fluoroquinolones if the ciprofloxacin MIC is 0.12 μg/ mL or higher even if the laboratory report identifies the isolate as susceptible. See https://emergency. cdc.gov/han/han00401.asp Vibrio cholerae Doxycyclined Ciprofloxacin, azithromycin, or ceftriaxone Non–Vibrio choleraed Usually not indicated for noninvasive disease. Single-agent therapy for noninvasive disease if treated.
Invasive disease: ceftriaxone plus doxycyclineUsually not indicated for noninvasive disease. Single-agent therapy for noninvasive disease if treated.
Invasive disease: TMP-SMX plus an aminoglycosideYersinia enterocolitica TMP-SMX Cefotaxime or ciprofloxacin Parasites Cryptosporidiumspp Nitazoxanide (HIV-uninfected, HIV-infected in combination with effective cART): Effective cART:
Immune reconstitution may lead to microbiologic and clinical response [154, 209, 210]NA Cyclospora cayetanensis TMP-SMX Nitazoxanide (limited data) Patients with HIV infection may require higher doses or longer durations of TMP-SMX treatment Giardia lamblia • Tinidazole
Note: Based on data from HIV-uninfected children
• NitazoxanideMetronidazole
Note: Based on data from HIV- uninfected children• Tinidazole is approved in the United States for children aged ≥3 years. It is available in tablets that can be crushed.
• Metronidazole has high frequency of gastrointestinal side effects. A pediatric suspension of metronidazole is not commercially available but can be compounded from tablets. Metronidazole is not FDA approved for the treatment of giardiasis.Cystoisospora belli TMP-SMX Pyrimethamine
Potential second-line alternatives:
• Ciprofloxacin
• NitazoxanideTrichinellaspp Albendazole Alternative: mebendazole • Therapy less effective in late stage of infection, when larvae encapsulate in muscle Fungus Microsporidia For disseminated (not ocular) and intestinal infection attributed to microsporidia other than Enterocytozoon bieneusi or Vittaforma corneae:
• Albendazole after initiation of cART and resolution of signs and symptoms
For E. bieneusior V. corneaeinfections:
• Fumagillin recommended for treatment of infections due to E. bieneusi in HIV-infected adultsNA Effective cART therapy:
• Immune reconstitution may lead to microbiologic and clinical response
• Fumagillin for systemic use is unavailable in the United States and data on dosing in children are unavailable.
• Consultation with an expert is recommended.Abbreviations: cART, combination antiretroviral therapy; CDI, Clostridium difficile infection; FDA, US Food and Drug Administration; HIV, human immunodeficiency virus; MIC, minimum inhibitory concentration; NA, not applicable; TMP-SMX, trimethoprim-sulfamethoxazole.
aFor information on susceptibility patterns in the United States, see the National Antimicrobial Resistance Monitoring System (NARMS; https://www.cdc.gov/narms). Susceptibility testing should be considered when a therapeutic agent is selected.
bIf invasive disease is suspected or confirmed, ceftriaxone is preferred over ciprofloxacin due to increasing resistance to ciprofloxacin.cMost clinical laboratories do not test for azithromycin susceptibility.
dPrimary therapy is aggressive rehydration; antibiotics are adjunctive therapy.
Table 7.Fluid and Nutritional Management of Diarrhea
Degree of Dehydrationa Rehydration Therapy Replacement of Losses During Maintenancec Mild to moderate dehydration Infantsb and children: ORS, 50–100 mL/kg over 3–4 hours
Adolescents and adults (≥30 kg): ORS, 2–4 LInfants and children:
<10 kg body weight: 60–120 mL ORS for each diarrheal stool or vomiting episode, up to ~500 mL/day
>10 kg body weight: 120–240 mL ORS for each diarrheal stool or vomiting episode; up to ~1 L/day
Adolescents and adults:
Ad libitum, up to ~2 L/day
Replace losses as above as long as diarrhea or vomiting continuesSevere dehydration Infants: Malnourished infants may benefit from smaller-volume, frequent boluses of 10 mL/kg body weight due to reduced capacity to increase cardiac output with larger volume resuscitation.
Children, adolescents, and adults: Intravenous isotonic crystalloid boluses, per current fluid resuscitation guidelines, until pulse, perfusion, and mental status return to normal. Adjust electrolytes and administer dextrose based on chemistry values. Administer up to 20 mL/kg body weight until pulse, perfusion, and mental status return to normal.Infants and children:
<10 kg body weight: 60–120 mL ORS for each diarrheal stool or vomiting episode, up to ~500 mL/day
>10 kg body weight: 120–240 mL ORS for each diarrheal stool or vomiting episode; up to ~1 L/day
Adolescents and adults:
Ad libitum, up to ~2 L/day
Replace losses as above as long as diarrhea or vomiting continue.
If unable to drink, administer either through a nasogastric tube or give 5% dextrose 0.25 normal saline solution with 20 mEq/L potassium chloride intravenously.Adapted from Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003; 52(RR-16):1–16 and World Health Organization. The treatment of diarrhoea: a manual for physicians and other senior health workers (https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5216a1.htm).
Low-osmolarity ORS can be given to all age groups, with any cause of diarrhea. It is safe in the presence of hypernatremia as well as hyponatremia (except when edema is present). Some commercially available formulations that can be used as ORS include Pedialyte Liters (Abbott Nutrition), CeraLyte (Cero Products), and Enfalac Lytren (Mead Johnson). Popular beverages that should not be used for rehydration include apple juice, Gatorade, and commercial soft drinks.
Abbreviation: ORS, oral rehydration solution.
aA variety of scales are available to grade the severity of dehydration in young children but no single, standard, validated method exists. Note that signs of dehydration may be masked when a child is hypernatremic.
bBreastfed infants should continue nursing throughout the illness.
cAfter rehydration is complete, maintenance fluids should be resumed along with an age-appropriate normal diet offered every 3–4 hours. Children previously receiving a lactose-containing formula can tolerate the same product in most instances. Diluted formula does not appear to confer any benefit.
[Organisms that Are Reportable]
All organisms listed in the table of Infectious Diseases Designated as Notifiable at the National Level (https://wwwn.cdc.gov/nndss/) should be reported. The CDC acts as a common repository for states and territories for collecting data and reporting of nationally notifiable diseases. Reports of occurrences of nationally notifiable diseases are transmitted to the CDC each week from the 50 US states, 2 cities (Washington, District of Columbia and New York, New York) and 5 territories (American Samoa, Commonwealth of Northern Mariana Islands, Guam, Puerto Rico, and the US Virgin Islands). Provisional data are published weekly in the Morbidity and Mortality Weekly Report; final data are published each year by the CDC in the annual “Summary of Notifiable Diseases, United States” [206].
The following 13 conditions, which are associated with infectious diarrhea, are included in the table of Infectious Diseases Designated as Notifiable at the National Level—United States, 2017 (https://wwwn.cdc.gov/nndss/conditions/notifiable/2017/):
- Campylobacteriosis
- Cholera
- Cryptosporidiosis
- Cyclosporiasis
- Giardiasis
- Hemolytic-uremic syndrome, postdiarrheal
- Salmonellosis
- Shiga toxin–producing Escherichia coli
- Shigellosis
- Trichinellosis (trichinosis)
- Typhoid fever
- Vibriosis
- Foodborne disease outbreak
Resources:
(1) The 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Clin Infect Dis. 2017 Oct 19. doi: 10.1093/cid/cix669. [Epub ahead of print]
(2) Here is a link to the excellent Infectious Diarrhea Podcast (32:02) that is a review of the 2017 Diarrhea Guidelines from the IDSA.
(3) A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Clin Infect Dis. 2018 Jun 28. doi: 10.1093/cid/ciy381. [Epub ahead of print]
(4) National Notifiable Diseases Surveillance System (NNDSS)
(5) 2017 National Notifiable Conditions
(6) Surveillance Case Definitions for Current and Historical Conditions