Dr. Siddhartha Mukherjee is leading a study to see if a ketogenic diet which lowers the body’s insulin level can enhance the effectiveness of Aliqopa [copanlisib], a PI3K inhibitor.*
*Link is to the drug’s mechanism of action on the manufacturer’s website.
The following are excerpts from the Guardian article, Resource (1) below:
A groundbreaking clinical trial on whether diet could boost the effectiveness of cancer drugs is set to be launched by one of the world’s leading oncologists.
The work, led by Siddhartha Mukherjee at Columbia University Medical Center in New York, will investigate whether a high-fat, low-carbohydrate diet could improve outcomes for patients with lymphoma and endometrial cancer.
The first cohort [of study patients], who will begin treatment in October, are lymphoma patients with cancers that have not responded to treatment. They will be followed by endometrial cancer patients, and the team is awaiting approval to treat women with drug-resistant breast cancer.
The patients will be treated with a licensed drug, Aliqopa, that has previously been shown to have limited effects in such patients. However, recent animal studies, including a paper this week in Nature from Mukherjee’s lab, suggest its effectiveness could be significantly boosted when combined with diet changes aimed at lowering insulin levels.
To achieve this, patients will be put on a so-called ketogenic diet (high in fat, low in carbohydrate, normal protein). “If you combine them with a diet which [keeps insulin low], all of a sudden these drugs become effective,” said Mukherjee, referring to the Nature study. “The diet really works like a drug.”
The team arrived at the idea after being involved in trials of drugs that, like Bayer’s Aliqopa [copanlisib]*, were designed to target one of the most common cancer mutations, called PI3K, that is present in up to 40% of breast cancers.
*Link is to the drug’s website resources including the product insert.
Despite major pharmaceutical industry investment a decade ago, none of the drugs aimed at PI3K have a major impact on survival rates. “There was an enormous amount of hype around these drugs, huge investments of the order of billions of dollars,” said Mukherjee.
Mukherjee and colleagues noticed that a high proportion of patients on one of the original trials had become diabetic, and after initially dismissing this as a drug side-effect, they began to investigate. They discovered the drug was interfering with one of the body’s main metabolic circuits, causing a spike in insulin production, which had the effect of reactivating the mutated genetic pathway that was helping cancer cells proliferate and spread.
“It may be the central reason we don’t get effectiveness,” said Mukherjee.
The trial will be among the first to investigate whether diet can be used to boost the effectiveness of drugs, but others are expected to follow in the next few years.
Here is a link to the abstract of the trial that Dr. Mukherhee’s group will be running: Suppression of insulin feedback enhances the efficacy of PI3K inhibitors [Abstract of the trial referenced in Resource (1)] and here are excerpts from the abstract:
Mutations in PIK3CA, encoding the insulin-activated phosphoinositide-3-kinase (PI3K), and loss of function mutations in PTEN, a phosphatase that degrades the phosphoinositide lipids generated by PI3K, are among the most frequent events in human cancers1,2. However, pharmacological inhibition of PI3K has resulted in variable clinical responses, raising the possibility of an inherent mechanism of resistance. Since the PIK3CA-encoded enzyme, p110α, mediates virtually all cellular responses to insulin, targeted inhibition of this enzyme disrupts glucose metabolism in multiple tissue types. . . . We hypothesized that insulin feedback induced by PI3K inhibitors may be reactivating the PI3K-mTOR signalling axis in tumours, compromising their effectiveness7,8. Here, we show in several model tumours, that systemic glucose-insulin feedback caused by targeted inhibition of this pathway is sufficient to activate PI3K signalling, even in the presence of PI3K inhibitors. We demonstrate that this insulin feedback can be prevented using dietary [ketogenic diet]* or pharmaceutical approaches, which greatly enhance the efficacy/toxicity ratios of these compounds. These findings have direct clinical implications for the multiple p110α inhibitors that are in clinical trials and provide a means to significantly increase treatment efficacy for patients with a myriad of tumour types.
*There are many flavors of ketogenic diet. It will be interesting to see the details of the ketogenic diet that the study is using. This should be in the methods part of the paper.
Resource (3), below, reviews the PIK3 inhibitors. Here are some excerpts:
Introduction
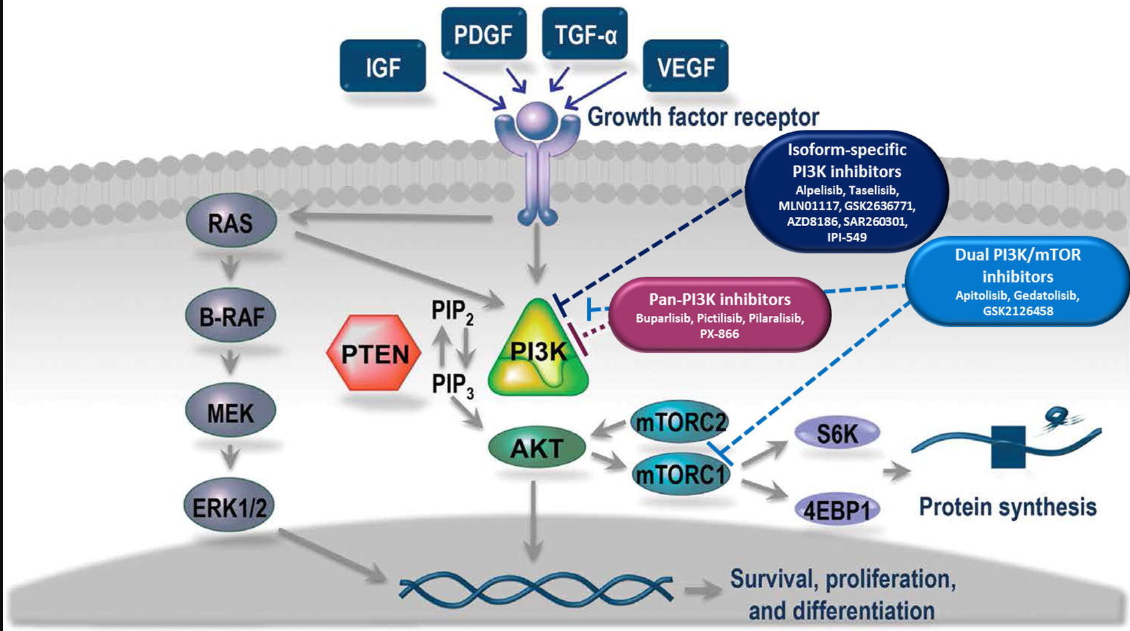
Phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway components are key therapeutic targets in cancer, immunity, and thrombosis [1]. In normal cells, the PI3K/mTOR pathway has regulatory roles in cell survival, proliferation, and differentiation [2]. However, aberrant activation of this pathway frequently occurs in human cancer [3]. Multiple inhibitors targeting key nodes within the PI3K pathway are in various stages of clinical development for the treatment of human malignancies [4].
Class I PI3Ks are of particular therapeutic interest, given their importance in the development of human cancers. Class I PI3Ks are heterodimers, comprising a catalytic (p110α, p110β, p110δ, or p110γ) and a regulatory (p85α, p55α, p50α, p85β, p55γ, or p101) subunit. Oncogenic PI3K/mTOR signaling is well characterized and has been reviewed in depth elsewhere [3].
Here is a link to Fig 1 above: Download high-res image (200KB)
Fig. 1. Mechanism of action of PI3K/mTOR inhibitors. Signaling through the PI3K pathway regulates multiple cellular processes that can contribute to the development of malignancies. Multiple inhibitors are under development that target PI3K and/or mTOR. Abbreviations: 4EBP1 = eIF4E-binding protein 1, ERK1/2 = extracellular signal-regulated kinase 1/2, IGF = insulin-like growth factor, MEK = mitogen-activated protein kinase kinase, mTOR = mammalian target of rapamycin, mTORC = mTOR complex, PDGF = platelet-derived growth factor, PI3K = phosphoinositide 3-kinase, PIP2 = phosphatidylinositol 4,5-bisphosphate, PIP3 = phosphatidylinositol 3,4,5-trisphosphate, PTEN = phosphate and tensin homolog, TGF-α = transforming growth factor-alpha, VEGF = vascular endothelial growth factor.
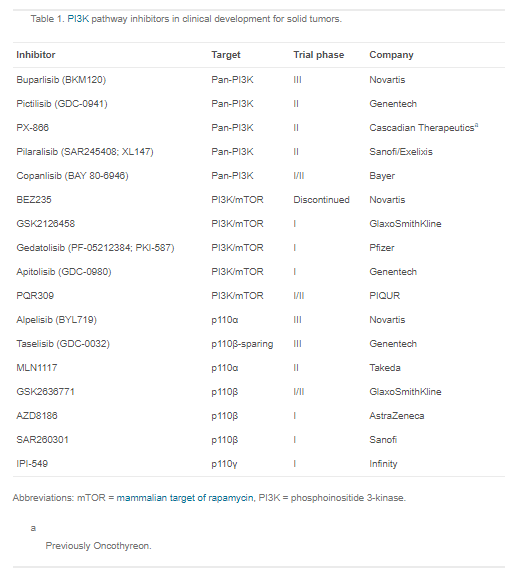
Table 1 below lists the PI3Kis discussed in this review. [Aliqopa (copanlisib) Link is to Bayer’s website.]
Resources (4) and (5) review what is known about the ketogenic diet (spoiler alert: apparently not a lot.
Resource (5), Rationale, Feasibility and Acceptability of Ketogenic Diet for Cancer Treatment, has in Table (1) in the article that gives details of the specific ketogenic diets used in the 10 studies they revieewed.
Resources:
(1) Top oncologist to study effect of diet on cancer drugs: Siddhartha Mukherjee says trial is first in a series on ‘rethinking human diets for cancer’. Fri 6 Jul 2018 18.00 BST, by Hannah Devlin Science correspondent, The Guardian (U.S. Edition).
(2) Suppression of insulin feedback enhances the efficacy of PI3K inhibitors [Abstract of the trial referenced in Resource (1)].
(3) Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: From laboratory to patients [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Cancer Treat Rev. 2017 Sep;59:93-101. doi: 10.1016/j.ctrv.2017.07.005. Epub 2017 Jul 18.
(4) Ketogenic diet in cancer therapy [PubMed Citation] [Full Text HTML] [Full Text PDF]. Aging (Albany NY). 2018 Feb 11;10(2):164-165. doi: 10.18632/aging.101382
(5) Rationale, Feasibility and Acceptability of Ketogenic Diet for Cancer Treatment [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Cancer Prev. 2017 Sep;22(3):127-134. doi: 10.15430/JCP.2017.22.3.127. Epub 2017 Sep 30.