Note to myself: In addition to today’s resource, please review:
- [The H2FPEF Score]: Links To And Excerpts From “A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction”
Posted on March 20, 2024 by Tom Wade MD - Links To And Excerpts From “Elevated Pulmonary Pressure Noted on Echocardiogram: A Simplified Approach to Next Steps”
Posted on January 8, 2024 by Tom Wade MD - Links To And Excerpts From “A Novel Method for Estimating Right Atrial Pressure With Point-of-Care Ultrasound”
Posted on December 16, 2023 by Tom Wade MD - Link To And Excerpts From “Echocardiographic evaluation of diastolic function in the setting of pulmonary hypertension”
Posted on March 22, 2024 by Tom Wade MD - 2023 ACC Expert Consensus on Cardiac Amyloidosis: Key Points
Jan 23, 2023 | Supriya Shore, MD - 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023;Jan 23: [Epub ahead of print]
- 2023 ACC Expert Consensus on Management of HFpEF: Key Points
Apr 19, 2023 | Supriya Shore, MD - 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023;Apr 19:[Epub ahead of print]
Today, I review, link to, and excerpt from The Curbsiders‘ #427 Kittleson Rules Amyloidosis.*
*Gorth DJ, Kittleson MM, Williams PN, Watto MF. “#427 Kittleson Rules Amyloidosis”. The Curbsiders Internal Medicine Podcast. thecurbsiders.com/category/curbsiders-podcast Final publishing date February 19, 2024.
The moderators, guest speaker, and show notes are outstanding, as usual.
All that follows is from the above resource.
Transcript available via YouTube
The clinical picture of an underrecognized disease
Amyloidosis demystified. Learn the clinical clues that suggest possible amyloidosis and how to order the correct tests to diagnose this disease. We are joined by Dr. Michelle Kittleson who shows us the ropes of treating fibril accumulation, @MKittlesonMD (Cedars Sinai).
Show Segments
- Introduction
- Case Presentation: Mr. Smith
- Differential Diagnosis of Heart Failure with Preserved Ejection Fraction
- [2023 ACC Expert Consensus on Management of HFpEF: Key Points
Apr 19, 2023 | Supriya Shore, MD]- [2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023;Apr 19:[Epub ahead of print]]
- Understanding Amyloidosis
- Clinical Clues and Red Flags for Amyloidosis
- Physical Examination Findings
- Workup for Amyloidosis
- Treatment Options
- AL Amyloidosis and Hematologist’s Role
- ATTR Amyloidosis and Cardiologist’s Role
- Other Therapies for TTR Amyloidosis
- Therapy on the Horizon
- Cost and Access to Tafamidis
- Alternative Therapies and Supplements
- Monitoring and Side Effects of Tafamidis
- Symptomatic Management and Anticoagulation
- Take Home Points
Amyloidosis Pearls
- Don’t stop at the diagnosis of HFpEF, figure out if there is an underlying cause for the patient’s disease.
- Amyloidosis is an under-recognized cause of HFpEF; a little more than 1 in 8 cases of HFpEF may be attributed to amyloidosis (González-Lopéz et al 2015).
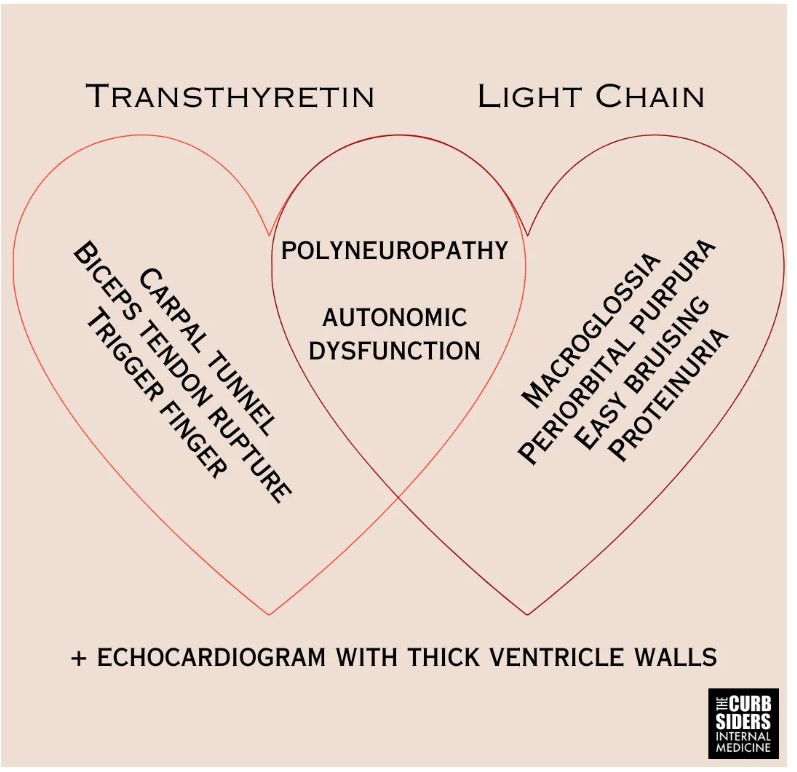
- Amyloidosis is caused by misfolded protein fibril aggregates accumulating in tissues. The two most common types of amyloidosis affecting the heart are due to misfolded monoclonal immunoglobulin light chains (L) or transthyretin (TTR).
- Fibril deposition in the heart leads to thick ventricle walls, and this infiltrative disease can cause persistent troponin elevation despite clean coronaries (Morioka et al 2022).
- Amyloid fibrils also deposit into nerves resulting in peripheral neuropathy, autonomic dysfunction, and erectile dysfunction.
- Fibril deposits (particularly TTR) into tendons result in tendinopathies like biceps tendon rupture and carpal tunnel syndrome–be particularly suspicious of bilateral carpal tunnel in individuals >60-years-old.
- Patients with amyloidosis often have orthostatic hypotension related to autonomic neuropathy (Palma et al 2019).
- A patient who has an echo with left ventricular wall thickness > 12mm and one red flag should get further workup (Garcia-Pavia et al 2021).
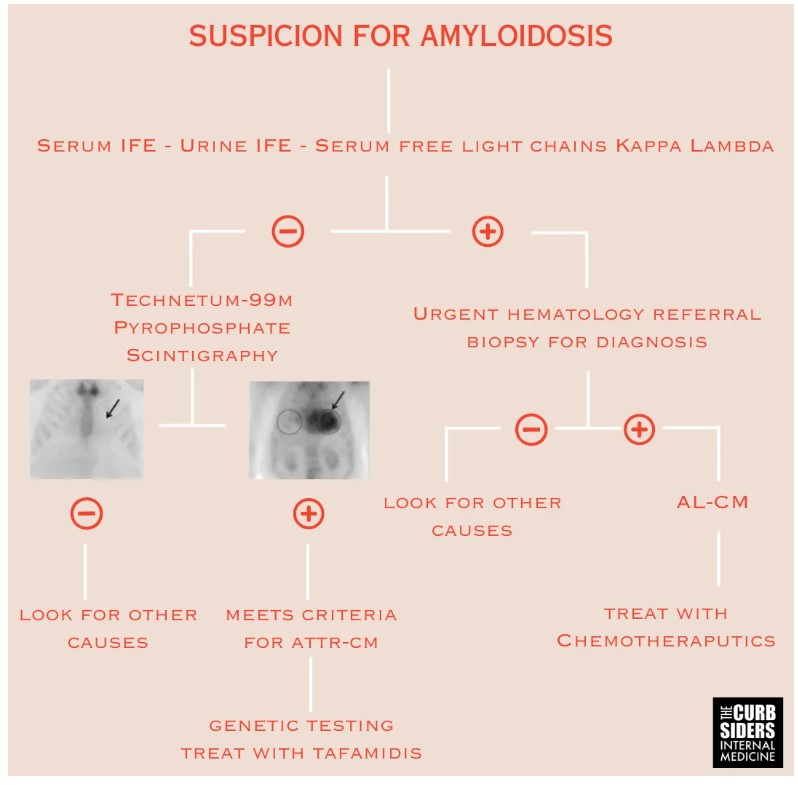
- First step of amyloidosis diagnosis is to exclude AL-CM with a monoclonal protein screen comprising serum immunofixation electrophoresis (IFE), urine IFE, and kappa/lambda free light chains.
- If the monoclonal protein screen is positive, the patient needs an urgent hematology referral to receive a biopsy for definitive diagnosis and address potential AL-CM.
- The second step is ordering a technetium-99m pyrophosphate (Tc-PYP) scintigraphy to look for ATTR-CM; a negative monoclonal protein screen and a positive Tc-PYP scan is sufficient to diagnose ATTR-CM (Kittleson et al 2023|ACC Expert Consensus).
- Treatment for AL-CM is driven by hematologists and comprises chemotherapies like daratumumab (anti-CD38), bortezomib (proteasome inhibitor), cyclophosphamide, and high dose dexamethasone (Bloom et al 2023).
- The only FDA approved treatment for ATTR cardiomyopathy is tafamidis (Kittleson et al 2023|ACC Expert Consensus).
- Anticoagulation is recommended for amyloidosis patients with atrial fibrillation, regardless of their CHADS-VASc score, due to the high thromboembolic risk associated with the disease (Garcia-Pavia et al 2021).
Amyloidosis
The differential diagnosis for dyspnea with edema does not stop with the heart. Heart failure with a preserved ejection fraction (HFpEF) is a diagnosis of exclusion; a patient with dyspnea and edema could be suffering from kidney pathology like nephrotic syndrome or liver pathology like cirrhosis causing portal hypertension or reduced protein metabolism. Amyloidosis is an under-recognized cause of HFpEF; a little more than 1 in 8 cases of HFpEF may be attributed to amyloidosis (González-Lopéz et al 2015). Cardiac amyloidosis should be carefully considered in patients with HFpEF, especially in patients without traditional risk factors like women, older individuals, chronic hypertension, coronary disease, diabetes, and chronic kidney disease (Lee et al 2020). Now that there is disease directed therapy for cardiac amyloidosis, don’t stop at the diagnosis of HFpEF, figure out if there is an underlying cause for the patient’s disease.
Pathophysiology and Nomenclature
Amyloidosis is caused by misfolded protein fibril aggregates accumulating in tissues. The two most common types of amyloidosis affecting the heart are due to misfolded monoclonal immunoglobulin light chains (L) or transthyretin (TTR). The nomenclature for amyloidosis includes an “A” for amyloidosis and a “CM” for cardiomyopathy. AL-CM occurs in the context of plasma cell dyscrasias, from monoclonal gammopathy of unknown significance (MGUS) to multiple myeloma. The protein transthyretin that causes ATTR-CM is a thyroid protein transporter also known as prealbumin, but it is not a synthetic precursor to albumin (Sanguinetti et al 2022). ATTR-CM can be caused by mutated transthyretin (ATTRv-CM) or wildtype protein (ATTRwt-CM), previously referred to as senile amyloidosis (Kittleson et al 2023|ACC Expert Consensus).
Past Medical History Concerning for Amyloidosis
Due to the tendency of amyloid fibrils to deposit in specific tissues (heart, nerves, and tendons), an amyloidosis diagnosis can unify seemingly disparate clinical findings. Fibril deposition in the heart leads to thick ventricle walls, and this infiltrative disease can cause persistent troponin elevation despite clean coronaries (Morioka et al 2022). Amyloid fibril deposition can also interfere with cardiac electrical conduction; unexplained atrioventricular block or prior pacemaker implantation can be clues suggesting the presence of amyloidosis (Hartnett et al 2021). To aid in your clinical decision making, there is a point-based risk score to identify patients with increased risk of ATTR-CM using 3 clinical parameters (age, male sex, and hypertension diagnosis) and 3 echocardiographic (echo) parameters (ejection fraction, posterior wall thickness, and relative wall thickness) (Davies et al 2022).*
*A Simple Score to Identify Increased Risk of Transthyretin Amyloid Cardiomyopathy in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2022;7(10):1036-1044. doi:10.1001/jamacardio.2022.1781
Kittleson pet peeve: increased left ventricular (LV) wall thickness on an echo does not mean hypertrophy; the increased thickness can be from an infiltrative disease like amyloidosis, and calling it hypertrophy can cause premature closure. Amyloid fibrils also deposit into nerves resulting in peripheral neuropathy, autonomic dysfunction, and erectile dysfunction. Patients with amyloidosis can have a .pronounced intolerance to vasodilating medications like angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), and calcium channel blockers (CCBs) (Kittleson et al 2023|ACC Expert Consensus). Fibril deposits into tendons result in tendinopathies. Carpal tunnel syndrome in adults older than 60–particularly bilateral carpal tunnel syndrome–is a red flag for amyloidosis (aus dem Siepen et al 2019 and Genova et al 2020). Other tendon manifestations include biceps tendon rupture (Geller et al 2017) and spinal stenosis (aus dem Siepen et al 2019). Amyloid tendinopathies can predate the development of symptomatic cardiac amyloid depositions by six or seven years and could be considered harbingers of disease (Perfetto et al 2022).
Physical Exam
ATTR-CM is a disease of older individuals, generally 60-years-old and above, with an insidious onset. AL-CM can occur in younger individuals, presenting more acutely than ATTR-CM. Due to autonomic dysfunction, patients with amyloidosis often have orthostatic hypotension (Palma et al 2019), a systolic decrease of 20 mmHg or a diastolic decrease of 10 mmHg within three minutes of standing. AL-CM is associated with macroglossia, easy bruising, and periorbital purpura (Hoffman et al 2020 and Bloom and Gorevic 2023). Additional cardiac findings are common, atrial fibrillation is present in around 70% of patients (Hartnett et al 2021), and there may be a systolic ejection murmur of aortic stenosis; ATTR-CM is present in 16% of patients with severe calcific AS undergoing TAVR (Castaño et al 2017).
Workup for Amyloidosis
There can be clues to a diagnosis of amyloidosis in common studies that your patient has likely already completed. An electrocardiogram (ECG) with low voltage suggests the presence of amyloid infiltration, or more accurately, discordantly low voltage for the left ventricular wall thickness (Garcia-Pavia et al 2021). Lab values changes include elevated BNP and troponin elevation despite clean coronaries (Morioka et al 2022). A patient who has an echo with left ventricular wall thickness > 12mm and one red flag should get further workup (Garcia-Pavia et al 2021).
First step of diagnosing amyloidosis is to exclude AL-CM with monoclonal protein screen, serum immunofixation electrophoresis (IFE), urine IFE, and lambda and kappa free light chains. IFE is more sensitive and specific than serum protein electrophoresis and should be preferentially used (Vermeersch et al 2008). If the monoclonal protein screen is positive, the patient needs an urgent hematology referral to address potential AL-CM. Definitive diagnosis of AL-CM requires a biopsy. It is ok to start with a surrogate site like a fat pad, but if that biopsy is negative, an affected organ biopsy of the heart or kidney will be necessary, as a surrogate site biopsies are insufficiently sensitive to exclude amyloidosis if there is a high clinical suspicion (Gillmore et al 2014).
If the monoclonal protein scan is negative, the next step is ordering a technetium-99m pyrophosphate (Tc-PYP) scintigraphy to look for ATTR-CM. It’s important to rule out AL-CM first, because AL-CM can cause a false positive Tc-PYP delaying appropriate treatment (Gillmore et al 2016). A negative monoclonal protein screen and a positive Tc-PYP scan is sufficient to diagnose ATTR-CM (Kittleson et al 2023|ACC Expert Consensus).
Once a patient is diagnosed with ATTR-CM, they should undergo genetic testing for variant forms of transthyretin. The most important variant to remember is Val124Ile; this variant is present in 3-4% of Black Americans (Chandrashekar et al 2012). Other common variants are Val30Met, Leu111Met, and Glu89Gln (Gentile et al 2023). Genetic testing in ATTR-CM affects available treatment options and triggers screening in first degree relatives. Relatives with a positive genetic screen should get an echo when they are 10-years-younger than the age when the index patient was diagnosed (Kittleson et al 2023|ACC Expert Consensus).