In this post I link to and excerpt from American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Blood Adv. 2020 Oct 13;4(19):4693-4738.
Here is the Abstract:
Background: Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), occurs in ;1 to 2 individuals per 1000 each year, corresponding to; 300 000 to 600 000 events in the United States annually.
Objective: These evidence-based guidelines from the American Society of Hematology (ASH) intend to support patients, clinicians, and others in decisions about treatment of VTE.
Methods: ASH formed a multidisciplinary guideline panel balanced to minimize potential bias from conflicts of interest. The McMaster University GRADE Centre supported the guideline development process, including updating or performing systematic evidence reviews. The panel prioritized clinical questions and outcomes according to their importance for clinicians and adult patients. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess evidence and make recommendations, which were subject to public comment.
Results: The panel agreed on 28 recommendations for the initial management of VTE, primary treatment, secondary prevention, and treatment of recurrent VTE events.
Conclusions: Strong recommendations include the use of thrombolytic therapy for patients with PE and hemodynamic compromise, use of an international normalized ratio (INR) range of 2.0 to 3.0 over a lower INR range for patients with VTE who use a vitamin K antagonist (VKA) for secondary prevention, and use of indefinite anticoagulation for patients with recurrent unprovoked VTE. Conditional recommendations include the preference for home treatment over hospital-based treatment for uncomplicated DVT and PE at low risk for complications and a preference for direct oral anticoagulants over VKA for primary treatment of VTE
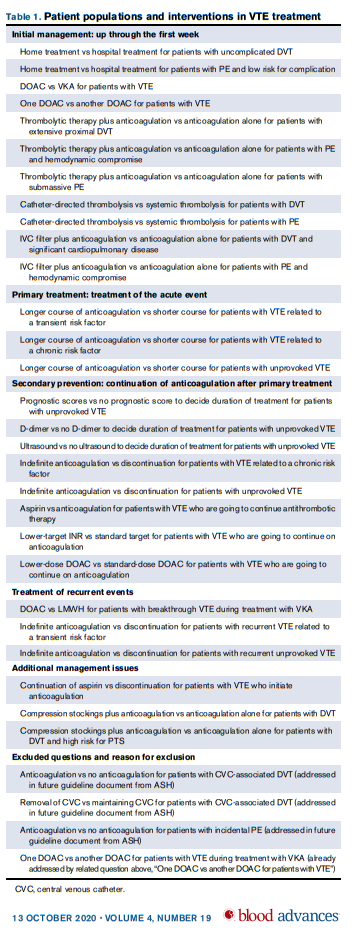
The Summary Of Recommendations lists all 28 recommendations with brief remarks for all recommendations.
The recommendations are divided into:
- Initial Management
- Recommendations 1 through 11
- Primary Treatment
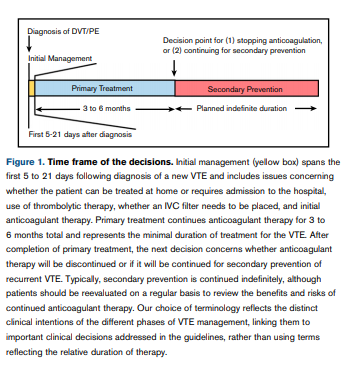
- “Primary treatment refers to the minimal length of time a patient must
be on therapeutic anticoagulation to treat the initial venous
thromboembolism (VTE) before consideration is given to discontinuing anticoagulation or switching to a long-term anticoagulation
regimen aimed at preventing VTE recurrence (secondary prevention) (Figure 1). Recommendations 12 through 14 refer to the
length of time for primary treatment of the initial VTE in 3 patient
populations.”
- “Primary treatment refers to the minimal length of time a patient must
- Secondary Prevention
- “Following completion of primary treatment for the initial VTE,
providers must decide whether to discontinue anticoagulant
therapy or continue with long-term anticoagulation with the intent
to prevent VTE recurrence, referred to as secondary prevention.
Recommendations 15 through 19 address which patients should
be considered for indefinite secondary prevention, and recommendations 20 through 22 address which antithrombotic therapies could be chosen for patients continuing indefinite secondary
prevention.”
- “Following completion of primary treatment for the initial VTE,
- Treatment of recurrent events
- Recommendations 23 through 25
- Additional Management Issues
- Recommendations 26 through 28
Introduction
Aim of the guideline and specific objectives
The purpose of this guideline is to provide evidence-based recommendations about the treatment of DVT and PE for patients without cancer. The target audience includes patients, hematologists, general practitioners, internists, hospitalists, vascular interventionalists, intensivists, other clinicians, pharmacists, and decision makers. Policy makers interested in these guidelines include those involved in developing local, national, or international programs aiming to reduce the incidence of VTE or to evaluate direct and indirect harms
and costs related to VTE. This document may also serve as the
basis for adaptation by local, regional, or national guideline panels.Description of the health problem
VTE, which includes DVT and PE, occurs in ∼ 1 to 2 individuals per 1000 each year, or ∼ 300 000 to 600 000 events in the United States annually.4 DVT most commonly occurs in the lower extremities but also affects the upper extremities.5,6 Approximately one third of all patients with a new diagnosis of VTE have PE, with or without DVT,7-9 and it is estimated that up to a quarter of all patients with PE present with sudden death.4
The risk for recurrent VTE varies according to whether the initial event was associated with an acquired risk factor, referred to as a provoked event, or in the absence of any provoking risk factors, referred to as an unprovoked event.10 For patients with unprovoked VTE, the risks of recurrent VTE after completing a course of anticoagulant therapy have been estimated to be 10% by 2 years and > 30% by 10 years.11,12 Long-term complications include PTS, which develops in 20% to 50% of patients after DVT and is severe in up to 5% of cases,13 and chronic thromboembolic pulmonary hypertension, which may develop in up to 5% of patients with PE.14
Anticoagulant therapy is very effective at preventing recurrent
VTE but is associated with an increased frequency of bleeding
complications. Major bleeding events may occur in ∼ 1% to 3% of patients on VKAs each year, compared to an ;30% lower relative risk for major bleeding with DOACs.15Description of the target populations
The incidence of VTE increases with age, ranging from ∼ 1 in 10 000 in individuals younger than 20 years of age to as high as ∼ 1 in 100 in individuals who are 80 years of age and older.16 VTE affects all races and ethnicities, with black persons having a higher incidence than white persons in most studies and individuals of Asian descent having a lower incidence than other races.17-19 Certain acquired characteristics identify subsets of individuals at higher risk for VTE, including individuals who are currently or were recently hospitalized, residents in
long-term care facilities, and patients undergoing surgical procedures.4Time frame of the decisions
Conceptually, the therapeutic management of patients with a new diagnosis of VTE can be divided into 3 phases: (1) initial management, which occurs from the time of diagnosis through the first 3 weeks of therapy; (2) primary treatment, which is a time-limited phase that typically runs for a minimum of 3 months; and (3) secondary prevention, which begins after completion of the primary treatment phase and extends for a prolonged, usually indefinite, period of time (Figure 1). The specific questions addressed by the guideline committee are most relevant at specific points in time during treatment, as summarized below.
Initial management
- Home treatment vs hospital treatment (Recommendations 1 and 2)
- Choice of anticoagulant therapy (Recommendations 3 and 4)
- Use of fibrinolytic therapy (Recommendations 5-9)
- Use of IVC filters (Recommendations 10 and 11)
Primary treatment
- Duration of primary treatment (Recommendations 12-14)
Secondary prevention
- Choice between stopping anticoagulation and indefinite therapy (Recommendations 15-19)
- Choice of treatment for secondary prevention (Recommendations 20-22)
The following sections represent topics that may occur during any phase of treatment
- Management of breakthrough and recurrent DVT/PE (Recommendations 23-25)
- Decision concerning use of aspirin while on anticoagulant therapy (Recommendation 26)
- Decision concerning use of compression stockings (Recommendations 27 and 28)