In this post, I link to and excerpt from Neurological manifestations of long-COVID syndrome: a narrative review [PubMed Abstract] [Full-Text HTML] [Full-Text PDF].
Therapeutic Advances in Chronic Disease. First Published February 17, 2022.
All that follows is from the above article.
Abstract
Accumulating evidence points toward a very high prevalence of prolonged neurological symptoms among coronavirus disease 2019 (COVID-19) survivors. To date, there are no solidified criteria for ‘long-COVID’ diagnosis. Nevertheless, ‘long-COVID’ is conceptualized as a multi-organ disorder with a wide spectrum of clinical manifestations that may be indicative of underlying pulmonary, cardiovascular, endocrine, hematologic, renal, gastrointestinal, dermatologic, immunological, psychiatric, or neurological disease. Involvement of the central or peripheral nervous system is noted in more than one-third of patients with antecedent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, while an approximately threefold higher incidence of neurological symptoms is recorded in observational studies including patient-reported data. The most frequent neurological manifestations of ‘long-COVID’ encompass fatigue; ‘brain fog’; headache; cognitive impairment; sleep, mood, smell, or taste disorders; myalgias; sensorimotor deficits; and dysautonomia. Although very limited evidence exists to date on the pathophysiological mechanisms implicated in the manifestation of ‘long-COVID’, neuroinflammatory and oxidative stress processes are thought to prevail in propagating neurological ‘long-COVID’ sequelae. In this narrative review, we sought to present a comprehensive overview of our current understanding of clinical features, risk factors, and pathophysiological processes of neurological ‘long-COVID’ sequelae. Moreover, we propose diagnostic and therapeutic algorithms that may aid in the prompt recognition and management of underlying causes of neurological symptoms that persist beyond the resolution of acute COVID-19. Furthermore, as causal treatments for ‘long-COVID’ are currently unavailable, we propose therapeutic approaches for symptom-oriented management of neurological ‘long-COVID’ symptoms. In addition, we emphasize that collaborative research initiatives are urgently needed to expedite the development of preventive and therapeutic strategies for neurological ‘long-COVID’ sequelae.
Introduction
Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, it has become evident that the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) would have immense repercussions on healthcare systems and socioeconomic structures worldwide.1 Besides the exorbitant death toll of the pandemic across the globe, with the rising number of COVID-19 survivors, increasing attention has been drawn to the prolonged or late-onset sequelae of SARS-CoV-2 infection, which are colloquially referred to as ‘long-COVID’ syndrome.2,
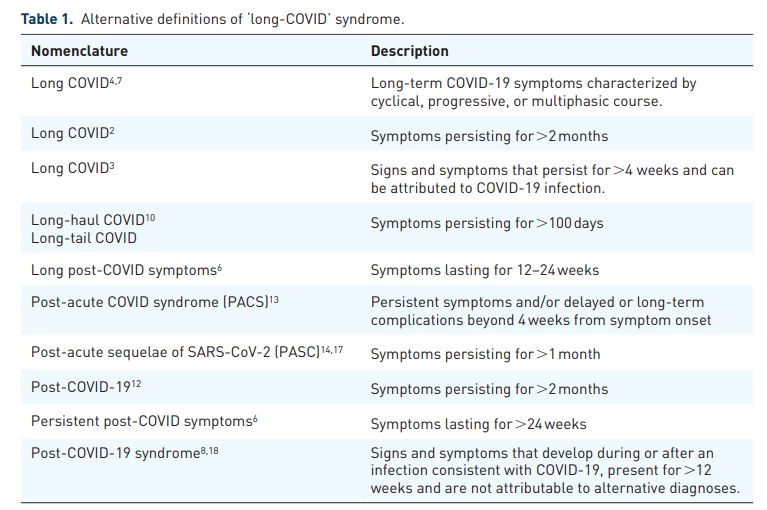
To enable better documentation and characterization of ‘long-COVID’, the World Health Organization (WHO) has recently assigned an emergency use International Classification of Diseases, Tenth Revision (ICD-10) code (U09.9) referring to ‘Post-COVID conditions, unspecified’.20 In addition, a clinical case definition was proposed by the WHO based on Delphi consensus, suggesting that ‘post COVID-19 occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis’. According to this definition,
Common symptoms include fatigue, shortness of breath, cognitive dysfunction but also others, and generally have an impact on everyday functioning. Symptoms may be of new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness, may fluctuate or relapse over time.18
Several limitations of this definition have been acknowledged, so far, including difficulties in approaching the diagnostic ‘time-windows‘ (especially in asymptomatic patients), the lack of robust clinical data to define clinical ‘cut-offs’, and the low specificity of proposed diagnostic criteria. Nevertheless, as more refined diagnostic criteria for ‘long-COVID’ are still underway, there is current consensus that the exclusion of acute COVID-19 should be regarded as a prerequisite for ‘long-COVID’ diagnosis.13
Neurological manifestations comprise one of the many facets of ‘long-COVID’ syndrome.21 In accord with the aforementioned definitions of ‘long-COVID’, the long-term or late-onset neurological sequelae of COVID-19 should be distinguished from the well-characterized acute neurological manifestations of SARS-CoV-2 infection5,22–24 Moreover, as ‘long-COVID’ is conceptualized as a multi-organ disease, central nervous system (CNS) and/or peripheral nervous system (PNS) involvement may present alone or in conjunction with pulmonary, cardiovascular, psychiatric, endocrine, hematologic, renal, gastrointestinal, dermatologic, or immunological symptoms.13,25 Similar to WHO, the National Institutes of Health (NIH) has linked ‘long-COVID’ to symptoms such as ‘fatigue, shortness of breath, “brain fog”, sleep disorders, fever, gastrointestinal symptoms, anxiety, and depression’, thereby acknowledging neurological symptoms as core aspects of ‘long-COVID’.26 Moreover, recent reports indicate an extremely high prevalence of long-term neurological manifestations among COVID-19 survivors, with nearly one-third of patients being diagnosed with neurological or psychiatric illnesses in the first 6 months following acute COVID-19.27
At present, neurologists are daily confronted with an increased demand for ‘long-COVID’ patient care. Yet, as scarce evidence has been consolidated so far, diagnosing and managing neurological complications of ‘long-COVID’ calls for navigation in ‘uncharted waters’. The aim of the present narrative review is thus to provide a comprehensive overview of our current understanding of the long-term neurological sequelae of COVID-19, along with a methodological framework for a systematic diagnostic approach and management of patients with neurological manifestations of ‘long-COVID’ syndrome.
Neurological manifestations of ‘long-COVID’ syndrome
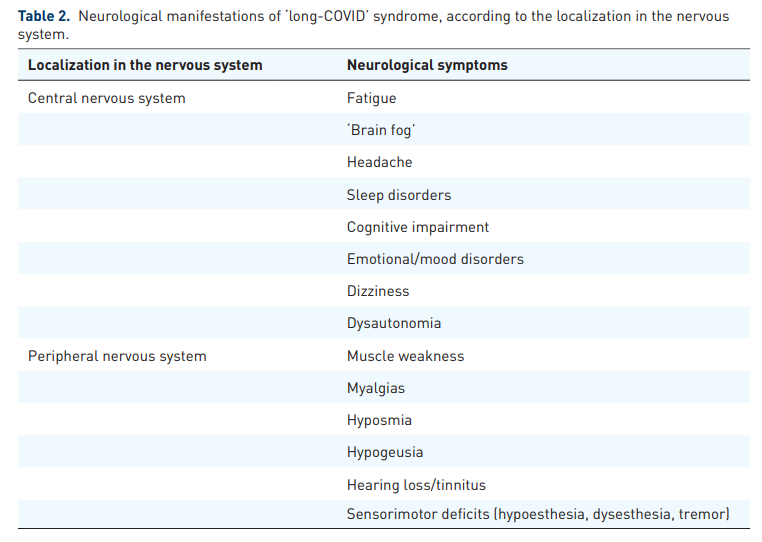
A wide array of neurological manifestations, involving both the CNS and PNS, have been described in the context of ‘long-COVID’ syndrome (Table 2).
It is important to emphasize, however, that neurological symptoms are often inextricable from ‘long COVID’manifestations that involve other organ systems, while nonspecific symptoms, including fatigue, ‘brain fog’, postexertional malaise, and sleep disorders, may comprise epiphenomena of underlying respiratory, cardiovascular, endocrine, renal, hematologic, autoimmune, or psychiatric diseases.8,25,29 As novel evidence continues to emerge, the spectrum of clinical characteristics of ‘long-COVID’ continues to widen. With respect to neurological manifestations, however, there is only scarce epidemiological evidence to date on the incidence of neurological disorders in populations with antecedent SARS-CoV-2 infection.30,31 Conversely, there are abundant data from observational studies, including evidence from patient-led research,32,33 reporting highly variable prevalence estimates of diverse neurological symptoms among ‘long-COVID’ patients.34–38 Due to inherent methodological caveats of most so-far published studies, including selection and reporting biases, lack of standardized neurological assessment, and lack of adjustment for comorbidities or concomitant ‘long-COVID’ manifestations in other organ systems, particular caution is warranted when attempting to characterize neurological sequelae of ‘long-COVID’.
While evidence from observational studies continues to accrue, a recently published systematic review and meta-analysis, including data from 47,910 patients, provided prevalence estimates for long-term COVID-19 effects.41 This meta-analysis revealed that 80% of patients infected with SARS-CoV-2 developed one or more long-lasting symptoms, with the most frequent being fatigue (58%), headache (44%), and attention disorder (27%). In addition, the following prevalence estimates for neurological manifestations were reported: ageusia (23%), anosmia (21%), memory loss (16%), hearing loss or tinnitus (15%), chills (7%), dizziness (3%), and stroke (3%). Crucially, psychiatric symptoms, including anxiety and depression, were observed in 13% and 12% of patients, respectively, while a lower prevalence was recorded for mood disorders, dysphoria, obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD), each affecting 2% of patients.41
Pathophysiological mechanisms underlying neurological manifestations of ‘long-COVID’
The pathophysiological processes implicated in CNS and PNS manifestations of acute COVID-19 have been extensively studied and reviewed in the scientific literature.22,23,42,43 Besides severe affection of the respiratory system, cardiovascular, renal, and gastrointestinal manifestations, including liver and pancreatic dysfunction, are well-characterized complications of acute COVID-19.44 In brief, several overlapping pathogenetic mechanisms of neurological manifestations of acute COVID-19 have been established, including viral neuroinvasion accompanied by aberrant neuroimmunological responses, endotheliopathy associated with blood–brain barrier dysfunction, coagulopathies that precipitate hypoxic–ischemic neuronal injury, metabolic imbalances, oxidative stress cascades, and cellular apoptosis (Figure 1).2,45–49
In contrast to acute neurological manifestations of COVID-19, however, the biological underpinnings of neurological ‘long-COVID’ sequelae remain today poorly characterized. In the absence of diagnostic markers and robust neuropathological data, most published articles have so far proposed putative pathophysiological mechanisms for neurological ‘long-COVID’ sequelae while drawing parallels with the pathophysiology of acute COVID-19.
The hypothesis of a predominant autonomic dysfunction in ‘long-COVID’ has been recently bolstered by findings of impaired regulation of heart rate variability (HRV) in ‘long-COVID’ patients.66
Concomitant involvement of other organ systems should also be considered when addressing the pathophysiology of neurological ‘long-COVID’ sequelae. While pathophysiological associations between nonspecific neurological symptoms, such as fatigue, ‘brain fog’, and postexertional malaise, and concomitant COVID-19-induced pulmonary or cardiac changes are well characterized,76,77 an involvement of the gastrointestinal tract and the brain–gut axis have also been recently proposed as a possible link to neurological manifestations of ‘long-COVID’.78,79 In particular, prolonged SARS-CoV-2 shedding has been observed in the gastrointestinal tract for up to 3 months postacute infection, with a recent study showing persistence of SARS-CoV-2 nucleic acids and proteins in 50% of patients who underwent intestinal biopsy.80 Bearing the role of brain–gut axis in the pathogenesis of neurodegenerative disorders in mind,81 further research is warranted to evaluate whether insidious gastrointestinal SARS-CoV-2 infection may be causally linked to neurological ‘long-COVID’ sequelae.
Finally, as a concluding remark on the pathophysiology of neurological manifestations of ‘long-COVID’, despite some ominous predictions expressed in the literature for perpetuation of neurodegeneration following SARS-CoV-2 infection, a recently published longitudinal study has indicated attenuation of CNS injury, despite the persistence of neurological symptoms at 6 months after acute COVID-19.82 In this study, the researchers measured biomarkers of astrocytic and neuronal injury, including neurofilament light-chain (NfL), glial fibrillary acidic protein (GFAp), and growth differentiation factor 15 (GDF-15), in 100 patients with antecedent SARS-CoV-2 infection and showed that despite significant elevation of these biomarkers’ concentrations during the acute phase of COVID-19, at 6-month follow-up, a normalization of all biomarkers was noted in all included patients. Nonetheless, one half of patients reported persistent neurological symptoms at 6 months, with the most frequent being fatigue in 40%, followed by ‘brain fog’ and cognitive changes in 29% and 25%, respectively. These data suggest that ongoing CNS injury may not necessarily accompany neurological ‘long-COVID’ sequelae82 and further indicate the pivotal role of a systematic approach for characterization, differential diagnosis, and management of ‘long-COVID’ symptoms. As acute COVID-19 has been associated with impaired neurotransmission and upper layer cortical circuitry dysfunction,83 a prolonged recovery of neurotransmission could underlie the prolonged neurological and cognitive deficits noted in ‘long-COVID’ patients.
Diagnostic approach to neurological ‘long-COVID’ sequelae
Taken together, the previous data reveal a very wide spectrum of neurological symptoms among COVID-19 survivors with only partially elucidated underlying pathophysiological mechanisms. There is thus a lot of ground still to be covered before diagnostic criteria for neurological ‘long-COVID’ sequelae can be solidified. In the absence of widely accepted operational definitions for ‘long-COVID’, the presence of symptoms, signs, or abnormal findings that persist beyond the resolution of acute COVID-19 and do not return to a premorbid baseline may be currently considered as long-term effects of the disease.41,84 As a consequence, a pragmatic approach to neurological sequelae of ‘long-COVID’ entails assessment of change from neurological baseline (Figure 2).
Besides the challenges in distinction of ‘true long-COVID’ from exacerbations or manifestations of new unrelated underlying disorders, some further aspects should be taken into account when approaching ‘long-COVID’ syndrome. First, there is currently lack of consensus on whether confirmed COVID-19 in the patient history or serological evidence of antecedent SARS-CoV-2 infection should be regarded as a prerequisite for ‘long-COVID’ diagnosis, as a substantial proportion of patients infected with SARS-CoV-2 remain asymptomatic or undiagnosed91,92 and highly variable seroprevalence rates have been reported during the post-COVID period,93–95 which are further confounded by serological responses to SARS-CoV-2 vaccines.96,97 Second, there is no consensus on an exact timeframe for defining ‘long-COVID’ in the literature. Some authors have proposed that ‘long-COVID’ may be considered after 2 weeks following acute SARS-CoV-2 infection,41,86 while a cut-off of 4 weeks after acute infection has been proposed by the CDC (Centers for Disease Control and Prevention).17
Conversely, the NICE (National Institute for Health and Care Excellence) guidelines distinguish between ‘ongoing symptomatic COVID-19’, which can be diagnosed in the period between 4 and 12 weeks postinfection, and ‘post-COVID-19 syndrome’, which may be diagnosed if symptoms persist beyond 12 weeks, with the latter definition also supported by the WHO (Table 1).19,18
Notwithstanding the aforementioned limitations in defining and approaching ‘long-COVID’, our understanding of long-term neurological sequelae of COVID-19 has begun to steadily expand. Emerging studies have provided promising evidence of improvement or even resolution of neurological symptoms, including hyposmia,116 anxiety/depression, cognitive impairment, fatigue, and overall functional status,117,118 at longer follow-up periods, up to 1 year following acute SARS-CoV-2 infection.
Therapeutic approach to neurological ‘long-COVID’ sequelae
Here, we propose a practical algorithm for diagnosis and management of patients presenting with neurological symptoms in the context of ‘long-COVID’ syndrome (Figure 2).
As previously discussed, nonspecific neurological symptoms may be causally linked to other persisting organ system dysfunction due to COVID-19, including respiratory, cardiovascular, psychiatric, endocrine, renal, hematologic, or autoimmune disease.8,25,29 Thus, early patient referral to other medical specialties and initiation of appropriate targeted treatments should be promptly considered.21
It is important to note that the use of standardized clinical, neurological, and functional scales is pivotal for initial patient assessment and follow-up.123–126
Moreover, particularly in patients presenting with fatigue, dyspnea, and postexertional malaise or autonomic dysfunction, assessment of oxygen saturation at rest and post exertion, performance of the 6-Minute Walk Test, and measurement of blood pressure (in lying position and while standing) are recommended.87,122,129
After initial clinical assessment, tailored ancillary testing, including blood tests, respiratory and cardiac function tests, neuroimaging, CSF and electrophysiological studies, may complement the neurological examination and should be decided on an individual patient basis.136 With respect to blood testing, assessment of full blood count, electrolytes, liver and renal function parameters, troponin, C-reactive protein, creatine kinase, D-dimer, brain natriuretic peptides, ferritin, and thyroid hormonal status has been recommended for initial patient screening.19,87 In addition, chest X-ray, electrocardiogram (ECG), and urine tests may be indicated depending on the findings of the clinical examination.87,122 Taken together, these recommendations underline that early identification and treatment of comorbidities comprise a cornerstone for the management of patients with neurological ‘long-COVID’ sequelae. Moreover, tailored neuroimaging and neurophysiological studies may be indicated for the exclusion of serious neurological CNS or PNS disorders, albeit previously published guidelines on ‘long-COVID’ emphasize that overinvestigation should be avoided.87,137
After excluding serious comorbidities or ongoing complications of COVID-19, management of neurological manifestations of ‘long-COVID’ should be pragmatic and symptom-oriented.21,87,137 To date, there is very limited evidence that pharmacological approaches could be effective in the treatment of neurological ‘long-COVID’ sequelae.29,87
Discussion
Conversely, based on the so-far published epidemiological data several inferences can be drawn. First, neurological ‘long-COVID’ sequelae, with clinically objectifiable correlates of CNS or PNS involvement, seem to affect at least one-third of patients with antecedent SARS-CoV-2 infection.27,40
Second, there is a substantial overlap between neurological and psychiatric ‘long-COVID’ symptoms. In a significant proportion of ‘long-COVID’ patients, however, concomitant respiratory, cardiovascular, endocrine, renal, hematologic, or autoimmune disease may underlie the manifestation of nonspecific neurological ‘long-COVID’ symptoms.8,25,29
Third, there is preliminary evidence indicating an increased risk of neurological long-term sequelae among patients with antecedent SARS-CoV-2 infection compared with patients infected with other respiratory tract viruses, including influenza but also other coronaviruses such as SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).27,151,152
In conclusion, neurologists are confronted today with an unprecedented need to comprehend and manage neurological ‘long-COVID’ sequelae.