Yesterday, I reviewed the outstanding Cribsiders‘ podcast and show notes, #40: Period Problems: Heavy Menstrual Bleeding. DECEMBER 8, 2021 By DR. SAM MASUR.
In the above resource, the guest expert, Dr. Weyand, states that a patient can have clinically significant with troubling but often non-specific symptoms from iron deficiency without anemia (meaning iron deficiency with a normal CBC).
A Google search of “Can you have iron deficiency without anemia?” revealed the following articles. Today I’ll excerpt from each.
- Iron deficiency without anemia – a clinical challenge [Pubmed Abstract] [Full-Text HTML] [Full-Text PDF]. Clin Case Rep. 2018 Jun; 6(6): 1082–1086.
- Iron deficiency without anemia: indications for treatment [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. 04/2020, Review. Gynecological and Reproductive Endocrinology and Metabolism.
- Iron deficiency without anaemia: a diagnosis that matters [PubMed Abstract] [Full-TextHTML] [Download Full-Text PDF]. Clin Med March 2021.
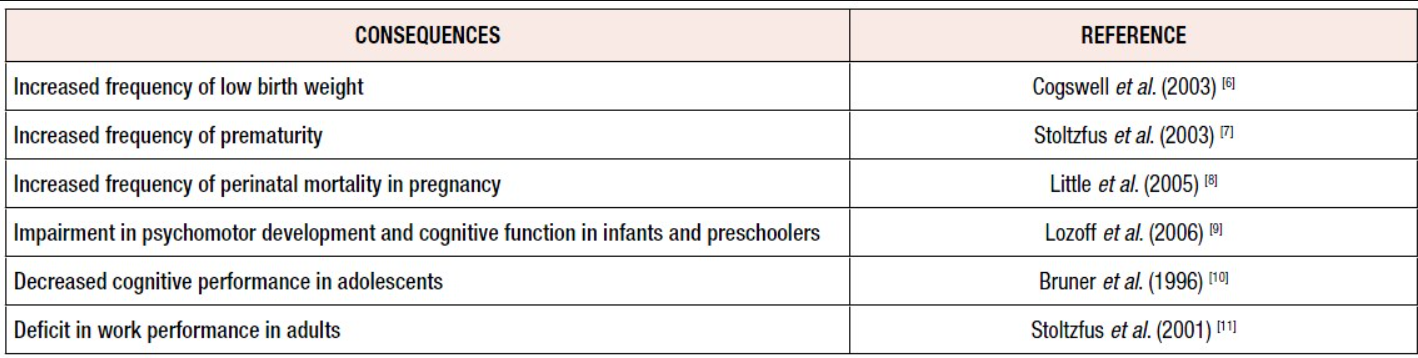
All that follows is from Iron deficiency without anemia – a clinical challenge [Pubmed Abstract] [Full-Text HTML] [Full-Text PDF]. Clin Case Rep. 2018 Jun; 6(6): 1082–1086.
Introduction
Iron deficiency may be severe despite a normal hemoglobin and full blood count. Symptoms which may be prolonged and debilitating, should raise a clinical suspicion on iron deficiency even if full blood count is normal. A lifelong history of blood loss, such as abundant menstruation, pregnancies, blood donations, accidents/ surgery as well as history of celiac disease, atrophic gastritis and drugs limiting gastric acid secretion, should be
taken.Ferritin (<30 lg/L) is most sensitive and specific indicator of iron deficiency, although its pitfalls need to be taken into consideration. However, the ferritin concentration may be near to normal, while iron staining of a bone marrow aspiration sample is devoid of iron.
Iron therapy should be monitored with repeated ferritin determinations with a target ferritin concentration of >100 lg/L and carried out until symptoms have resolved. When iron treatment is discontinued, the serum ferritin should be determined to ensure that the level remains stable. Iron should be reinstituted if the ferritin concentration drops and symptoms reappear.
Clinical Challenge
Iron deficiency anemia (hemoglobin ≤ 130 g/L in males and ≤120 g/L in females) is a late manifestation of iron deficiency, both of which are common medical conditions in everyday clinical practice [2–4]. Some 10–20% of menstruating women have iron deficiency, and 3–5% of them are frankly anemic [4]. Iron deficiency (anemia) may often be asymptomatic and go undiagnosed for a long period of time.
[When the role of ferritin in the diagnosis of iron deficiency is unclear,] Then the ferritin can be supplemented with
determination of transferrin saturation, soluble transferrin
receptor (sTfr) and the ratio between sTfr and logarithm
of ferritin as well as hepcidin [2, 3].When is the ferritin concentration abnormal?
The question of which ferritin concentration signifies
clinically symptomatic iron deficiency has been raised [5,
6]. Patients with true iron deficiency anemia on the basis
of negative bone marrow iron staining may have a serum
ferritin concentration close to 50 lg/L [7]. Patients with
the restless leg syndrome should be considered iron deficient when their ferritin concentration is <75 lg/L [8].
Furthermore, patients with negative bone marrow iron
stores have been shown to present with serum ferritin
levels of close to 100 lg/L [9]. Iron deficiency in patients
with heart failure impairs the quality of life, irrespective
of the presence of anemia. There, iron deficiency was
defined as a ferritin level <100 lg/L or, at 100–299 lg/L
with transferrin saturation <20%, [10]. Notably patients
with inflammation or clinically significantly impaired liver
or renal function were excluded from the trial [10].Symptoms and differential diagnosis
Weakness, fatigue, difficulty in concentrating, and poor
work productivity are nonspecific symptoms ascribed to
low delivery of oxygen to body tissues and decreased
activity of iron-containing enzymes [2, 3, 11–13]. The
extent to which these non-hematologic effects of iron
deficiency are manifested before anemia develops may be
unclear [2–4].During my 30-year carrier as an internist with a special interest in thyroid diseases and hematology, I have met hundreds of patients, mainly menstruating females, who seek advice because of prolonged (1–25 years) fatigue, brain fog, muscle and joint pains, weight gain, headache, dyspnoea, palpitations, sometimes associated with sleep disturbances, arrhythmia, lump in the throat or difficulty in swallowing, and restless legs. The patients have often received a spectrum of diagnoses,
such as subclinical hypothyroidism (treated with
levothyroxine alone or with T3 containing preparations), chronic fatigue syndrome, fibromyalgia, chronic
Lyme disease, burnout, and overtraining. The blood
count has usually been normal. At referral, their serum
ferritin concentrations have ranged from 1 to about
150 lg/L. If there has been no obvious reason for iron deficiency, such as celiac disease, multiple blood donations, multiple pregnancies, or long periods of abundant menstruation, the differential diagnostics at serum ferritin >50 lg/L has included liver and kidneys diseases, occult blood in stools, IgA deficiency, calcium disorders, D-vitamin or vitamin B12 deficiency and, if
the history revealed prolonged use of pyridoxine (>20 mg/daily, vitamin B6 toxicity [14].Cases highlighting the Problem
In the article, the author describes two instructive cases.
Conclusion
The presented cases with symptoms of iron deficiency anemia as examples are demonstrating that a normal full blood count is common in association with low ferritin levels that would indicate iron deficiency. It is much more important to listen to the patient’s description of his/her symptoms than to use the full blood count to rule out iron deficiency. If symptoms are in accordance with iron deficiency, the patient should be considered iron deficient at least up to a serum ferritin concentration of 100 lg/L, or even much higher, if the patient has an inflammatory condition, kidney disease or fatty liver [2, 3]. Iron deficiency irrespective of manifestation should always be treated (17).
The ferritin level should be controlled regularly during and after the iron administration with a sustained target ferritin of more than 100 lg/L. A marked improvement or total disappearance of symptoms should decide the duration of iron treatment. If the patient has apparently had iron deficiency for more than [5]–10 years, the ferritin concentration may repeatedly drop with the reappearance of symptoms when oral (Fig. 1) or intravenous
(Fig. 2) iron therapy is discontinued.When the patient is symptomless he/she should be followed for an extended period of time to ascertain that the ferritin concentration remains stabilized, especially if the patient is a female with abundant menstruations or planning pregnancy. No blood donations should be allowed. I consider the diagnosis and management of iron deficiency without anemia as one of the greatest challenges during my 35-year career as an internist. Furthermore, I am convinced that there is still a lot to be discovered about iron metabolism.
All that follows is from Iron deficiency without anemia: indications for treatment [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. 04/2020, Review. Gynecological and Reproductive Endocrinology and Metabolism.
INTRODUCTION
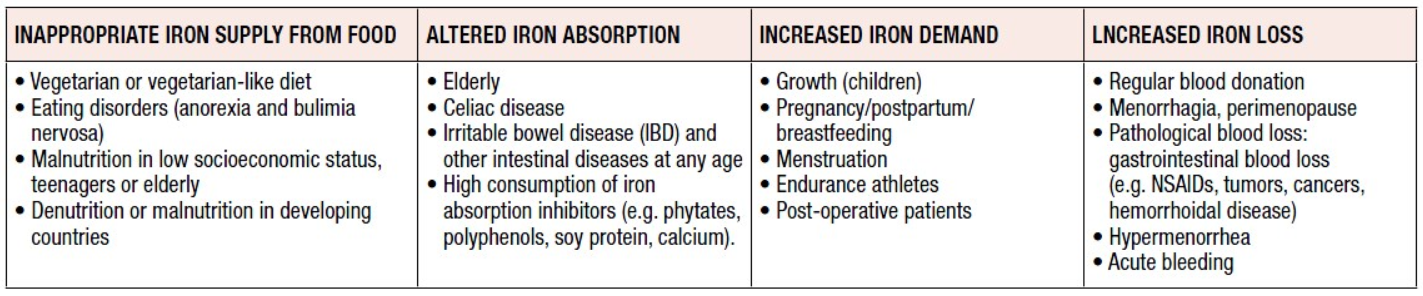
lron deficiency (ID) occurs when absorption of dietary iron is not sufficient to cover loss of iron and the body’s iron requirements. The most severe form of ID is iron deficiency anemia (IDA), which develops after depletion of physiological iron stores. IDA is the most common nutritional deficiency worldwide, occurring in 1.24 billion people, mostly women and children in developing countries [1]. The prevalence of iron deficiency without anemia (IDWA) is unknown although it has been suggested that it can be twice that of IDA [2]. Iron deficiency anemia is due to diets that are poor in bioavailable iron, and therefore inadequate to meet iron requirements. This problem is particularly important in high-risk groups like women and children [3-5]. Those at risk of ID may have one or a combination of many risk factor(s) (Table 1).
Recent studies have indicated that anemia per se is less important from a public health standpoint than the effects on health associated with tissue ID, as ID can have serious consequences (Table 2) [6-11].
In developed countries such as the USA and countries in Europe, ID is less frequent and less severe, with IDA occurring in 1 to 3% of adult men and post-menopausal women [12,13]. lron deficiency without anemia (IDWA) predominates. In this form of ID, a slightly negative iron balance reduces the body’s iron stores but the hemoglobin (Hb) concentration remains normal. lron deficiency without anemia is perceived to be a less severe, milder form of ID, although there are conflicting reports concerning its symptoms.
However, there is increasing evidence that ID both with and without anemia should be treated with iron supplementation. In this review the clinical consequences of depleted iron stores and the treatment of ID, particularly IDWA, are discussed.
Medical consequences of IDWA
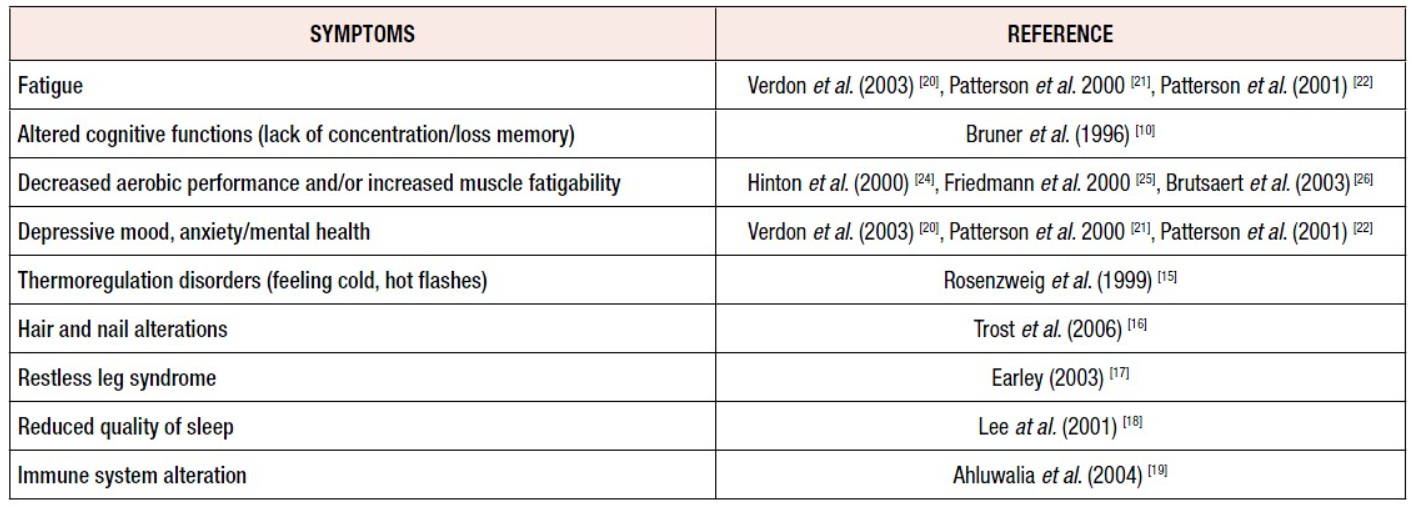
The evidence indicates that even mild ID (serum ferritin <20-35 ug/L) can result in symptoms such as fatigue, altered cognitive functions, decreased aerobic performance, restless legs syndrome and reduced quality of sleep [15-18]. Symptoms that may be related to IDWA, such as lowered physical work capacity or mental changes diagnosed by general practitioners, are not always specific (Table 3) [10,15-22,24-26].
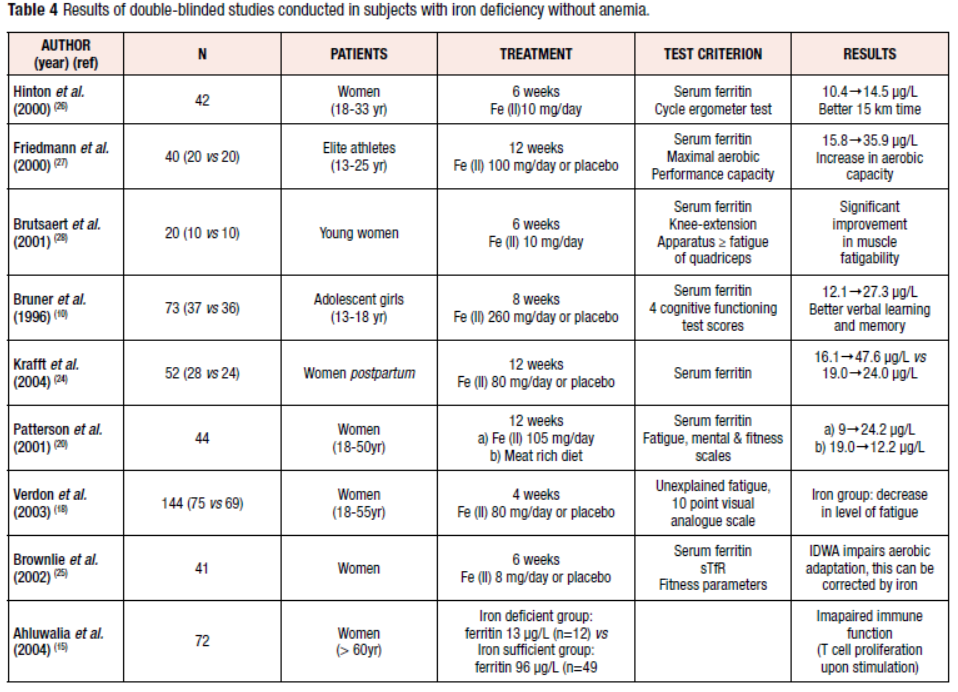
The relationship with IDWA is therefore best analyzed according to changes in symptoms after double-blind treatments with iron and placebo over a sufficiently long period. Several intervention studies have been performed in subjects with IDWA (Table 4) [10,18-20, 22-26].
Effects of IDWA on pregnancy and lactation
There are no clear data concerning IDWA during pregnancy and lactation and its relationship with feto-maternal morbidity. However, it is known that during pregnancy, early ID (first trimester) without anemia will result in anemia beginning around the second trimester (maximal red cell expansion) if no supplements are given [27]. In other words, preconceptional ID is a major risk factor for developing anemia in pregnancy and for its numerous negative effects. Iron requirements during pregnancy are double those of the pre-pregnancy period, due not only to the red blood expansion, but also to the fetal and placental development [28]. lt is known that neonates of woman with low or empty iron stores at term have lower iron stores during neonatal growth and adolescence. Iron supplementation should be routinely indicated during pregnancy unless iron stores of about 500mg (serum ferritin >70ug/L) are present at the beginning of pregnancy (achieved by approximately 15-20% of women in Western countries) [28]. The benefits of iron supplementation postpartum are indicated in a study by Krafft et al. [29]. Lactating women with low iron stores show lower iron transfer to the milk. Furthermore, ID in early pregnancy causes increased placental angiogenesis, which results in a so-called feto-placental miss ratio, an underlying cause of diseases after birth (fetal programming) [30]. Currently, other consequences of pure ID during pregnancy and lactation on mothers’ physical or mental performance are poorly investigated [31].
Fatigue and mental and somatic health and their relationship with iron deficiency
Fatigue is an unspecific symptom, responsive to iron therapy, that has been documented for more than 150 years [17,20,22, 32,33]. In a study by Verdon et al., 144 non-anemic women aged 18-55 years (mean serum ferritin 30 µg/L) received either 80 mg Fe(II)/day or placebo for four weeks [20]. The level of fatigue decreased significantly in the iron-treated group. A subgroup analysis showed that only women with ferritin <50 µg/L improved with oral supplementation. In a study conducted in Australia, 44 women with IDWA (serum ferritin <20 µg/L) were randomly allocated to either iron supplementation or a high-iron diet for 12 weeks (Table 4) [22]. Mental health and vitality scores increased in both intervention groups; ferritin increased more under iron supplementation.
The possible relationship between IDWA and mental and somatic health symptoms like anger or fatigue were studied by Sawada et al. [34] in a group of young Japanese young women (18-22 years). These authors concluded that fatigability, anger and tension were significantly higher in IDWA subjects than in normal subjects. These women also showed a higher proportion of neurotic tendencies. These findings may suggest that IDWA could be a risk factor for the development of anger or fatigue in young women [34]. In all these studies, various parameters of physical or mental health were clearly connected with IDWA. It is particularly worth noting that some of these unspecific symptoms improved after treatment with oral iron.
The immune system is related to iron status
Another critical function that could be impaired in mildly iron-deficient individuals is immune function. In a study by Ahluwalia et al. [19], 72 homebound, elderly, apparently healthy women provided blood for comprehensive evaluation of iron status and cell-mediated and innate immunity. Women were classified as iron deficient on the basis of multiple abnormal iron status test results. In iron-deficient women, T cell proliferation upon stimulation with concanavalin A and phytohemagglutinin A amounted to only 40-50% of that found in iron-sufficient women. lt was concluded that ID is associated with impairments in cell-mediated and innate immunity and may render older adults more vulnerable to infections.
Treatment options
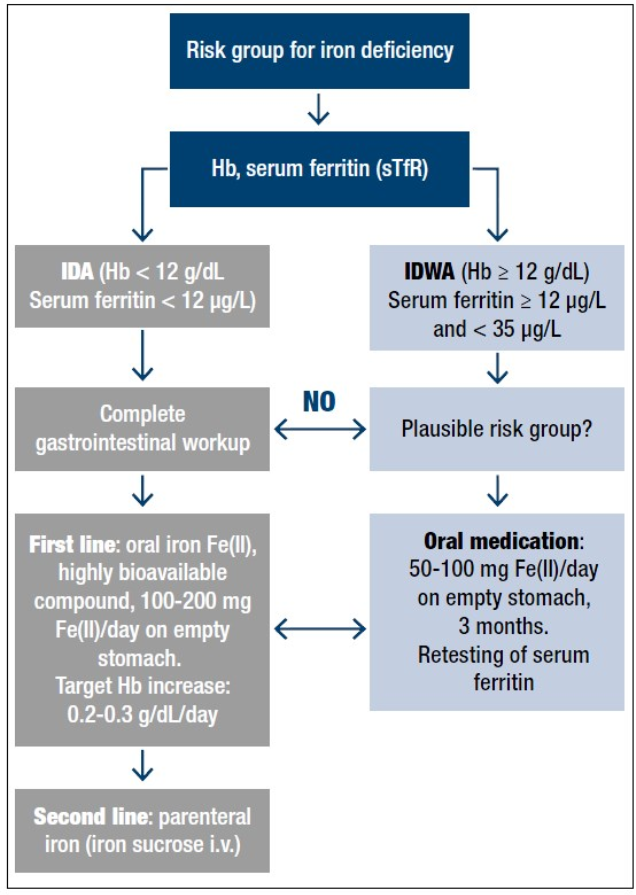
There is good evidence suggesting that every case diagnosed with ID, especially symptomatic patients, should be adequately treated with iron, even in the absence of anemia [35] (Figure 1).
The treatment should always be initiated following medical consultation, and every severe case of ID should be regarded as a serious clinical symptom that requires diagnostic evaluation of the underlying disease. Because daily iron loss in adults is in the range of 1-2 mg/day, moderate to severe IDA resulting in a deficit of 1000 to 2500 mg iron is, in most cases, due to abnormal blood loss (hypermenorrhea or gastrointestinal bleeding) [14]. Therefore, in all these patients, a complete gastrointestinal evaluation including colonoscopy and gastroduodenoscopy should be obligatory. [Emphasis added]
In IDWA (Hb 12 g/dl; serum ferritin >12 µg/L), as discussed above, there are good arguments for treatment with oral iron for at least 3 months in order to replete the iron stores completely, i.e. to reach a serum ferritin value of ideally >50 µg/L. The lower cut-off value of serum ferritin for intervention with iron supplementation is less well defined. Some authors hesitated to give iron to individuals with serum ferritin <20 µg/L. Others take <35 µg/L or, in the elderly even <50 µg/L, as an indication of partly depleted iron stores which are already having physiological consequences [20].
Treatment with oral ferrous iron preparations is known to be effective and safe in most patients. Despite much progress also with parenteral iron preparations, oral iron therapy is clearly the first-line treatment in all subjects with diagnosed ID, because of safety aspects. Among oral iron preparations, the use of any ferrous salt (mostly ferrous sulfate) is generally recommended in the literature, albeit without much discussion of the relevance of the bioavailability of a given pharmaceutical iron preparation.
The efficacy and also the frequency of side effects depend upon the administered daily dose and the application modus. In adults, a typical dose of 60-100 mg/day Fe is normally sufficient to increase the Hb concentration by 0.2-0.3 g/dl/day in a non-bleeding anemic patients [27, 38- 40]. In patients with substantial blood loss (hypermenorrhea, gastrointestinal blood loss), up to 200 mg of elemental iron/day in 2-4 fractions is possible without serious side effects [41]. The best bioavailability is found when iron is taken on an empty stomach together with water. This can be stabilized by combination with fruit juice (ascorbic acid), whereas the use of tea, milk, cola, coffee or cereals should be avoided as these contain substances that act as inhibitors of iron absorption [42-44]. lf administered together with a meal, iron supplements may be better tolerated in some individuals, but at the cost of substantially decreased bioavailability [42].
In conclusion, when iron is used and carefully monitored in individuals with ID, the “iron hypothesis” should not be relevant. This is shown in Figure 2 with a u-type risk profile. The goal is to normalize the body’s iron stores in both respects. The high risk on the right side of the figure with very severe iron overload should not be a reason for neglecting the risk of ID on the left side, which should be treated with the appropriate iron formulation.
CONCLUSIONS AND PERSPECTIVES
IDWA is an under-recognised, underdiagnosed and certainly undertreated entity. The balance of current evidence strongly indicates that to ensure optimal health and development, it is important to prevent and treat even mild ID not only in growing individuals and menstruating and pregnant women, but also in endurance athletes and the elderly.
Considering the ingenious systems available that control the absorption and metabolism of iron, it is paradoxical that ID is still the most common deficiency disorder in the world and the main remaining deficiency in the developed world. A reasonable explanation may be the marked changes that have occurred in human nutrition. The current low-energy lifestyle has further led to a situation where the risk of nutrient deficiencies, especially ID, have increased. IDWA is not easy to diagnose, and any chosen cut-off value of diagnostic parameters of iron status (serum ferritin, sTfR) will have either low specificity or low sensitivity. Serum ferritin remains the diagnostic gold standard in practice. Values below 20-35 µg/L make ID the most likely diagnosis and do at the same time exclude substantial iron overload. lt is therefore very important in practice that iron supplementation is always based on actual serum ferritin values in order to prevent inappropriate doses from being administered. Bearing this in mind, all the risk profiles for inducing iron overload by iron supplementation are irrelevant. Several intervention studies show that iron supplementation in subjects with IDWA is effective and helpful for the patient. It may also prove cost-effective for the healthcare system, as further diagnostic costs are minimized. Nevertheless, parallel to iron supplementation, the cause of IDWA should be carefully explored. Faced with a patient outside a classical risk group for ID, the possibility of occult blood loss should always be related to the development of ID. lron deficiency without anemia could be a transient stage to severe ID, in which a complete gastrointestinal workup is necessary in order to find the reason. The first-line treatment of ID is oral treatment using a pharmaceutical ferrous iron preparation with documented high bioavailability. Repletion of exhausted iron stores using a 60-100 mg dose of ferrous iron (Fe(ll))/day on an empty stomach can take up to 3 months. Retesting serum ferritin (>50 µg/L) can document the therapeutic success. Treatment failure is caused by low compliance (e.g. side effects), the wrong iron compound (e.g. low bioavailability), or a suboptimal administration modus (e.g. consumed with iron-chelating food ingredients).
All that follows is from Iron deficiency without anaemia: a diagnosis that matters [PubMed Abstract] [Full-TextHTML] [Download Full-Text PDF]. Clin Med March 2021.
ABSTRACT
Iron deficiency anaemia (IDA) currently affects 1.2 billion people and iron deficiency without anaemia (IDWA) is at least twice as common. IDWA is poorly recognised by clinicians despite its high prevalence, probably because of suboptimal screening recommendations. Diagnosing IDWA relies on a combination of tests, including haemoglobin and ferritin levels, as well as transferrin saturation. Although the causes of iron deficiency may sometimes be obvious, many tend to be overlooked. Iron sufficiency throughout pregnancy is necessary for maternal and foetal health. Preoperative IDWA must be corrected to reduce the risk of transfusion and postoperative anaemia. Oral iron is the first-line treatment for managing IDWA; however, intravenous supplementation should be used in chronic inflammatory conditions and when oral therapy is poorly tolerated or ineffective. This review considers the causes and clinical features of IDWA, calls for greater awareness of the condition, and proposes diagnostic and management algorithms.
Introduction
Iron deficiency (ID) is the most prevalent nutritional deficiency and a major precipitant of anaemia. According to a major international study, nearly 1.2 billion people suffer from iron deficiency anaemia (IDA) and iron deficiency without anaemia (IDWA) is estimated to be at least twice as common.1,2 IDA is the most frequent presentation of ID; hence, there is an ongoing misconception that the two terms are synonymous. ID is a broader term and refers to low iron stores that do not meet the body’s iron requirements, regardless of whether anaemia is present or not.2 . . . Nevertheless, symptoms of anaemia such as fatigue can be present without anaemic haemoglobin levels.4 Recognising IDWA as a clinical diagnosis is crucial to ensuring adequate management, especially for patients with chronic conditions such as heart failure (HF) where IDWA can increase long-term mortality.5
Diagnostic definition of iron deficiency
Ferritin is an indicator of iron stores and is the most sensitive and specific biomarker for assessing ID. The WHO defines low ferritin as levels <15 μg/L for adults and <12 μg/L for children.6 However, in clinical practice, when ferritin levels dip below 30 μg/L, ID can be ascertained.7
Ferritin is an acute-phase reactant that is increased in serum during chronic inflammation. Cut-off values for ferritin in ID are increased to 100 μg/L in states of chronic inflammation.
Transferrin saturation (TSAT) levels below 20% are also diagnostic of ID. In chronic inflammatory conditions when ferritin levels are 100–300 μg/L, TSAT should be used to diagnose ID.6,8
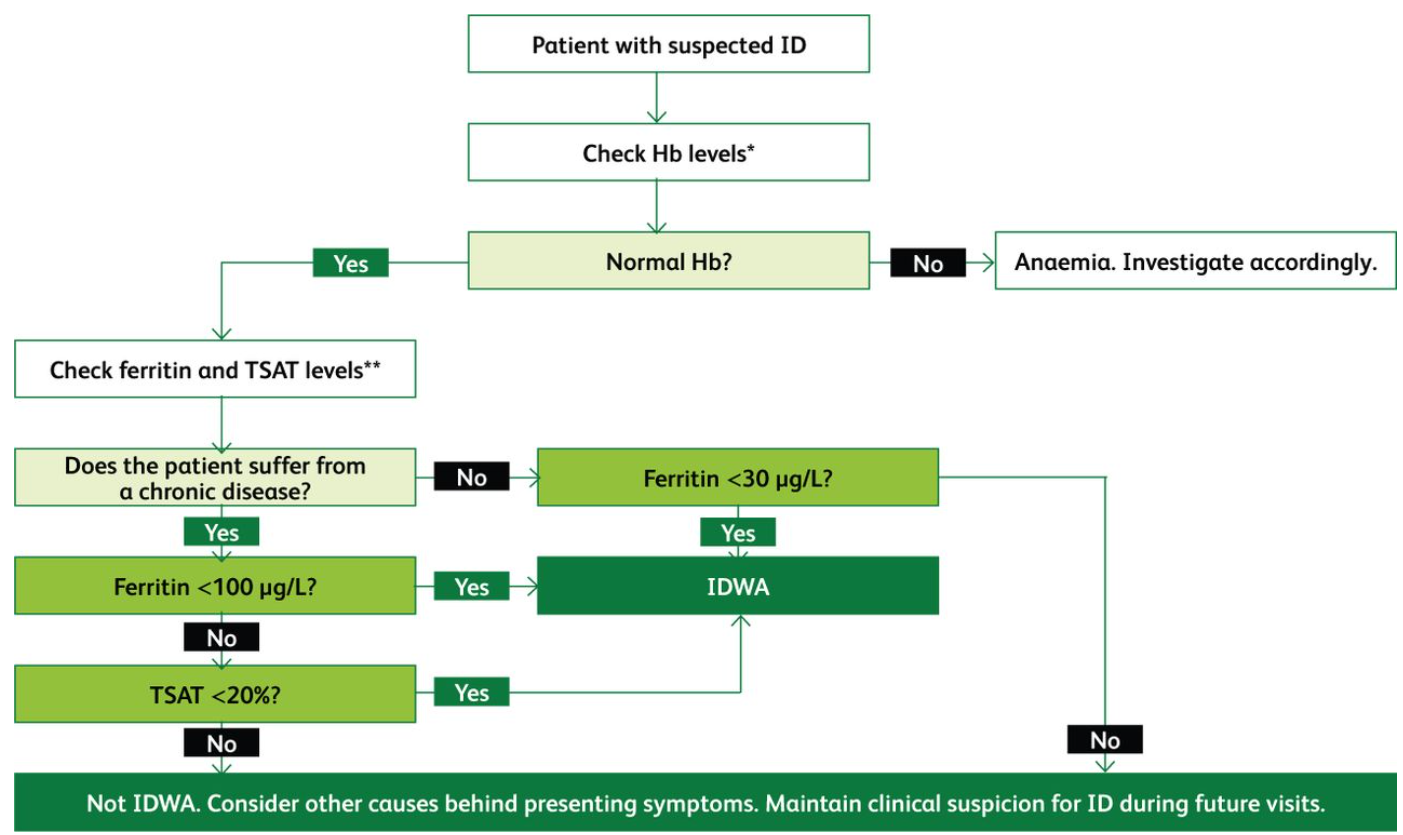
Serum iron levels fluctuate throughout the day and should not be used for diagnosis.9 Fig 1 proposes an algorithm for diagnosing ID based on the current literature.
An algorithm for the diagnosis of iron deficiency based on the best current evidence. This represents how tests are interpreted and not the order of requesting them.3,8,10 Hb = haemoglobin; ID = iron deficiency; IDWA = iron deficiency without anaemia; TSAT = transferrin saturation. *Normal levels of haemoglobin are ≥130 g/L for males, ≥120 g/L for females and ≥110 g/L for a pregnant female. **Ferritin levels below 30 μg/L indicate ID; in chronic inflammatory conditions ferritin levels may be elevated and so the threshold is raised to 100 μg/L. Ferritin levels can be raised to 100–300 μg/L in chronic inflammation; in such cases TSAT levels must be used. Normal ranges can slightly vary according to the laboratory.
Other useful tests include hepcidin, soluble transferrin receptor (sTFR) and reticulocyte haemoglobin content (RHC); however, they are not widely used. Although hepcidin is usually low or normal in absolute ID (AID), it helps distinguish AID from functional ID (FID).10 The sTFR is a valuable indicator of ID as, unlike ferritin, it is unaffected by inflammation. Unfortunately, performing this test takes too long and it is not widely available. When haemoglobin levels are normal, a low RHC indicates early ID in functional stores and hints at iron need, pre-anaemia and a risk of developing IDA.11
The distinction between IDA and IDWA relies on the use of strict haemoglobin cut-offs. However, clinicians should consider the fact that normal haemoglobin ranges have been set using population data. Essentially, what may be a normal haemoglobin level for one person may be abnormal for another, especially if a patient has a haemoglobin level in the low normal range but their usual haemoglobin levels are higher. Patients suffering from ID should be treated regardless of whether they are explicitly defined as anaemic. Cut-off haemoglobin ranges are useful, but their limitations should be kept in mind and patients should be assessed on a case-by-case basis.
Causes of iron deficiency
Iron has both a storage pool and a functional pool. The storage pool is the reticuloendothelial system which consists of the liver, spleen and lymph nodes. The functional pool consists of red blood cells, bone marrow and cardiac and skeletal muscle. Iron is absorbed in the duodenum via specific transporters and is carried by transferrin molecules to the storage and functional pools. Iron deficiency can be absolute or functional.
AID is when the storage pool is iron-deficient due to reduced intake, increased needs, reduced absorption or excessive loss. AID also causes low iron levels within the functional pool.
In FID the burden is the chronic inflammation, causing cytokine and hepcidin release. Hepcidin causes iron deficiency via the blockage of an iron exporter known as ferroportin. There are two ways in which this blockage causes ID. First, it reduces iron absorption in the duodenum; second, it causes iron retention within the storage pools. This means that despite normal iron levels within the storage pools, functional pools are iron deficient and cannot utilise the stored iron for vital body processes.2,10,12
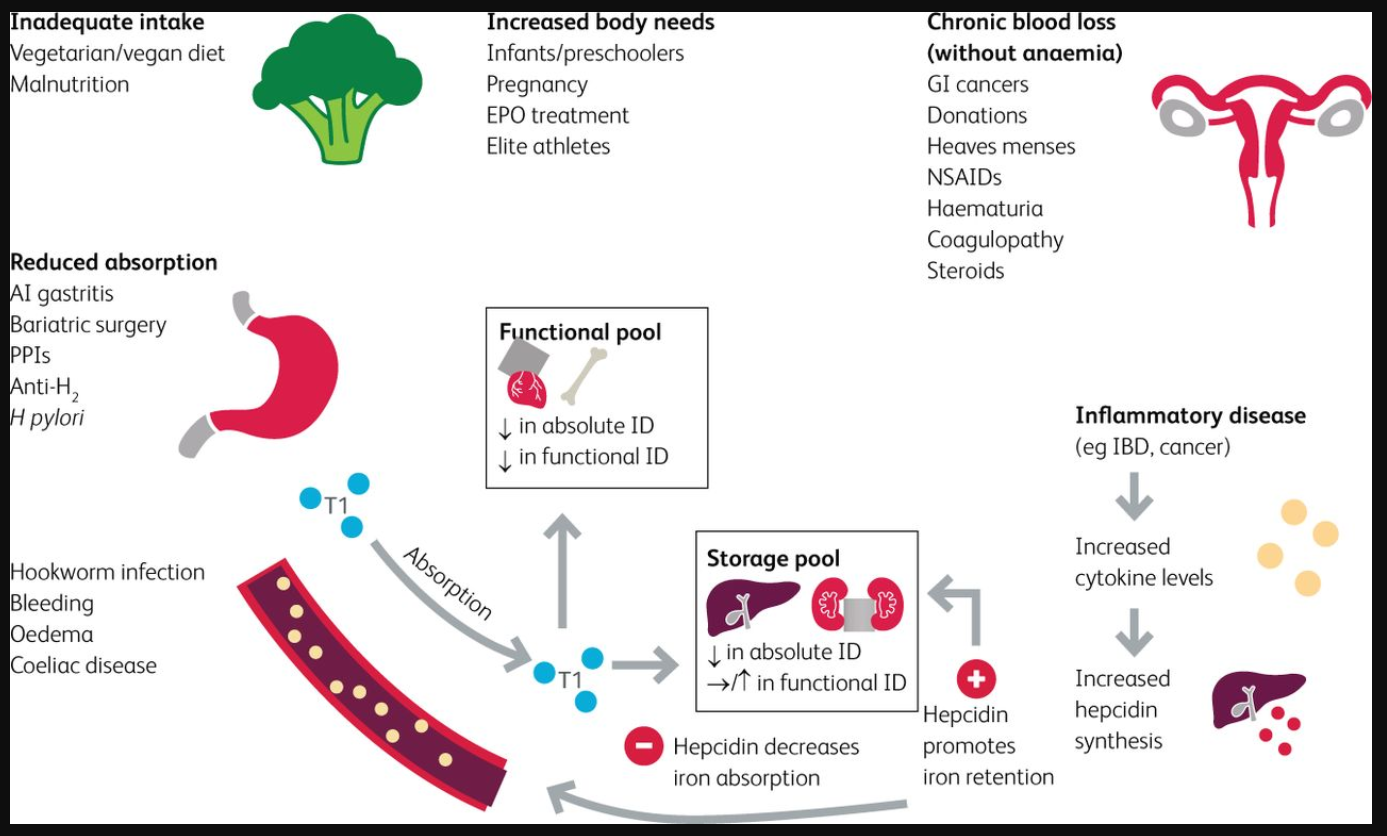
Causes of iron deficiency can be grouped into the following categories: inadequate dietary intake, increased body needs, reduced absorption, chronic inflammation and chronic blood loss.
Fig 2 summarises the causes of ID and provides a clinically relevant background of the pathophysiology at play.
Fig 2.
Causes of iron deficiency.2,10,25 AI gastritis = autoimmune gastritis; anti H2 = anti histamine-2 receptor (H2 receptor antagonist); EPO = erythropoietin; GI cancers = gastrointestinal cancers; H pylori = Helicobacter pylori; IBD = inflammatory bowel disease; NSAIDs = non-steroidal anti-inflammatory drugs; PPIs = proton pump inhibitors; Tf = transferrin.
Clinical features
It is well established that iron plays an indispensable role in haemoglobin and myoglobin synthesis. Less appreciated, however, is its role in mitochondrial functioning, including the synthesis of cofactors and enzymes necessary for cellular respiration.26,27 Consequently, highly metabolic cells such as cardiomyocytes and skeletal muscle cells are dependent on iron for optimum functioning.10 A randomised controlled trial (RCT) by Melenovsky et al showed that ID reduces aerobic respiration and citric acid cycle enzyme activity in advanced HF.28 Similarly, several studies have demonstrated the negative impact of ID on cellular metabolism.28–31 A recent RCT of 40 chronic HF patients with ID demonstrated enhanced skeletal muscle energetics following iron supplementation.31 Although many cellular processes depend on iron, it is likely that they are only impacted in severe ID, where anaemia would likely be present. More research is needed to further clarify the effects of IDWA on cellular processes and how such effects would relate to the clinical presentation.
Symptoms of ID such as fatigue and exercise intolerance are nonspecific, making it difficult to identify whether ID is the culprit or a chronic disease such as HF, which may present with similar symptoms.32 Other causes such as hypothyroidism, depression and burnout may also be responsible. Additionally, it is difficult to distinguish IDWA from IDA based solely on symptoms given their overlap; the main clinical difference is that symptoms are more severe in IDA. A recent systematic review concluded that iron supplementation in IDWA improves subjective measures of fatigue.4 In IDWA patients with coexisting HF or IBD, intravenous (IV) supplementation improves symptom control and quality of life, respectively.32,33 However, evidence on the effect of iron supplementation on physical activity, often assessed by maximal oxygen consumption tests (VO2 max), is mixed.15,34
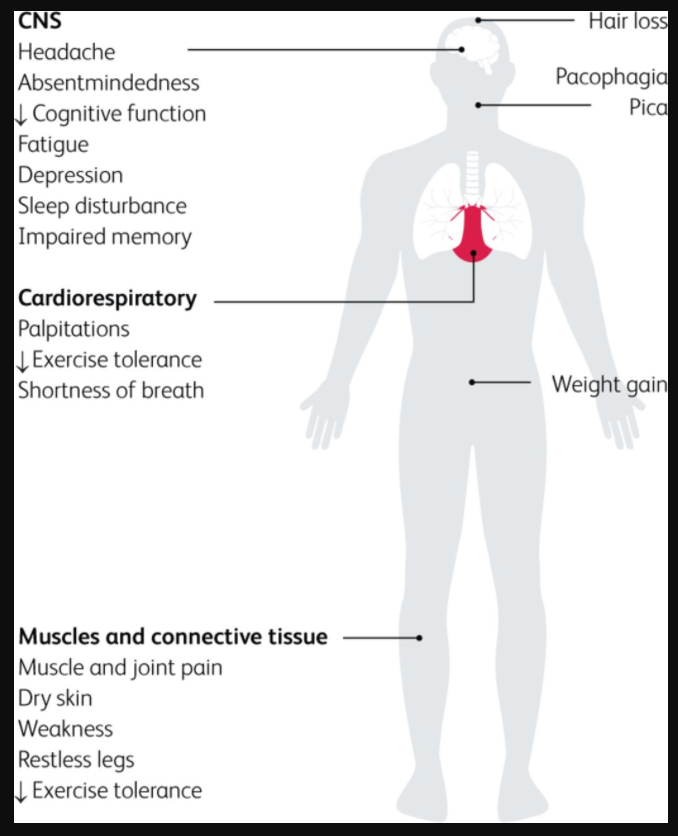
Severe ID may also cause cardiac and skeletal myopathy, which is detrimental in HF.35 This is due to impaired clearance of reactive oxygen species, increasing oxidative stress and thereby weakening cardiac muscle. Ultimately, the atrophy of cardiac, peripheral, and respiratory muscles leads to reduced exercise tolerance and dyspnoea on exertion.36 In HF, mitochondrial function is already impaired, and the superimposed effect of ID can be deleterious.37 The clinical features of IDWA are summarised in Fig 3.
Fig 3.
Effects of iron deficiency on the human body.13
Iron deficiency in pregnancy
IDA increases maternal morbidity and the risk of poor pregnancy outcomes, including intrauterine growth restriction, prematurity and low birth weight.38 Compared to non-iron-deficient women, gravidas with depleted iron stores or IDWA at the start of pregnancy are more likely to develop pre- and postnatal ID and have a newborn with a lower birth weight.39 Anaemia is a late manifestation of ID. During foetal growth and when iron is scarce, iron is directed primarily to erythropoietic tissues over the rest of the body. Therefore, ID may exist in other organs such as the brain, despite normal haemoglobin levels. ID has been associated with mental illness and impaired neurocognitive functions, such as poor memory and slower neural processing, which may be due to ID irrespective of anaemia.40 Postnatal ID is linked to neonatal iron status and foetal iron loading, and is associated with permanent cognitive and behavioural effects that are measurable until up to 19 years of age, even with postnatal iron repletion.41 Iron sufficiency is vital throughout the entire pregnancy. It is especially crucial from week 32 of gestation when rapid myelination of the brain begins and throughout infancy.42 Consequently, mothers should be screened and treated for ID before conception. Iron can be replaced throughout pregnancy using oral iron every other day in the first trimester to improve maternal absorption. If ID persists, then IV iron is safe to use in the second and third trimesters. Furthermore, newborns should be screened and treated for ID after birth to avoid permanent neurocognitive damage.38
Preoperative iron deficiency
The British Committee for Standards in Haematology recommends iron supplementation in patients with IDWA (ferritin <100 μg/L and TSAT <20%) who are planned to undergo surgery with a predicted haemoglobin loss of >30 g/L.47
An international consensus statement on perioperative ID and anaemia emphasised the importance of screening for and managing IDWA prior to surgery. Elective surgery with significant expected blood loss should be postponed until ID and/or anaemia is corrected to reduce the risk of postoperative anaemia. The recommendation is that oral iron is used when surgery is more than 6 weeks away; otherwise intravenous supplementation is best.48 Collaboration between primary and secondary care practitioners is vital to effective management of preoperative IDWA. Ideally, testing should occur in primary care when a referral is first made to avoid delays to surgery.47,48
Directions for management
IDWA should be treated when identified, with a target ferritin of 100 mg/L.7 Treatment should be continued until ferritin levels have normalised and symptoms have resolved. Patients should be offered dietary advice and oral iron replacement.15 IV replacement should be considered for symptomatic patients with treatment-resistant IDWA. Furthermore, ferritin levels should be checked every 6–12 months following treatment, especially in heavily menstruating women and those considering pregnancy.13
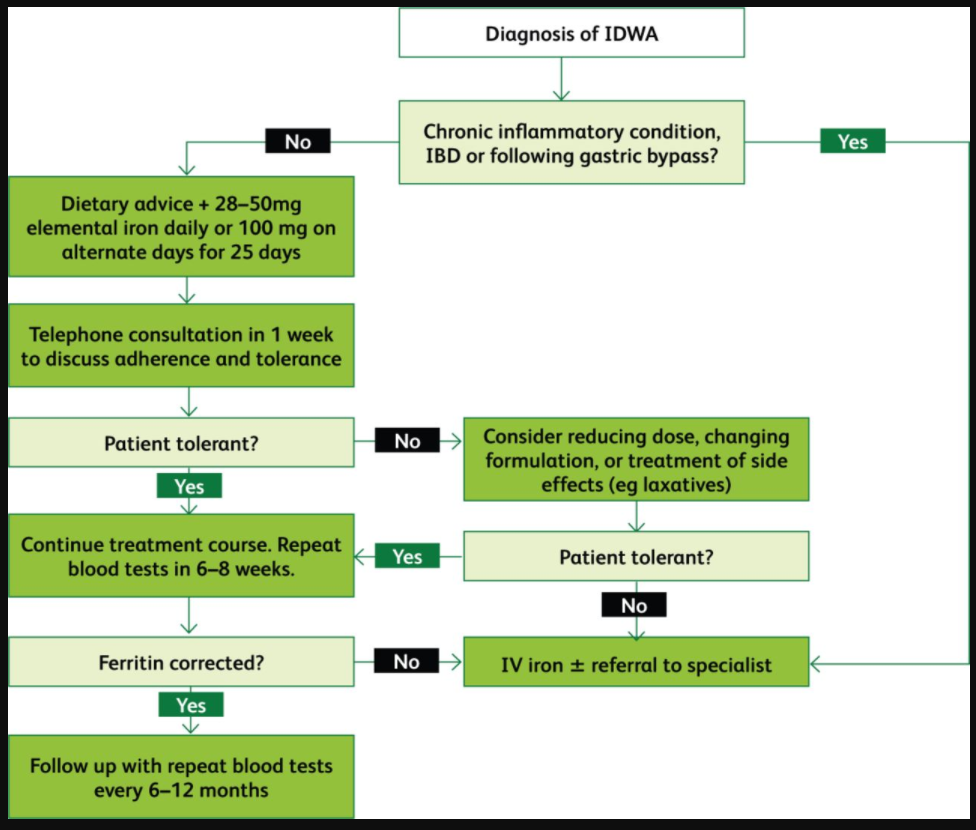
Oral iron is associated with gastrointestinal side effects such as constipation, diarrhoea, dyspepsia, and nausea, which have been associated with poor adherence.50 Using single doses on alternate days as opposed to multiple doses on consecutive days has been shown to result in higher absorption and better regulation of hepcidin levels in iron-depleted women.51 The recommended oral dose is 28–50 mg iron daily or 100 mg on alternate days for 25 days.15,49,52 Ideally, patients should be contacted 1 week following the start of treatment to assess drug tolerance, with changes to formulation or dose agreed if necessary.49 Patients’ haemoglobin, ferritin, and CRP levels in addition to red blood cell indices should be checked 6–8 weeks following the start of treatment to assess treatment response.15 If oral supplementation is inadequate, patients should be considered for IV replacement and referral to a specialist. Once ferritin levels have corrected, patients should be followed up with blood tests every 6–12 months, with replacement reintroduced if necessary.15
Where oral iron is ineffective or poorly tolerated, patients should be offered IV supplementation.53 It should also be used in IDWA to circumvent diminished absorption following gastric bypass, in IBD where the intestinal mucosa is damaged, and also in other chronic inflammatory conditions, including HF, where hepcidin is elevated.53–55 IV iron has proven to be effective and well-tolerated in IDWA, and may be more efficacious than oral preparations.52,56 Around 1% of patients experience minor infusion reactions, which should prompt cessation of infusion and management of symptoms (see Gómez-Ramírez et al for detailed guidance).57 Severe reactions have traditionally been overstated due to the risks associated with high molecular weight iron dextran (HMWID), which has now been replaced by substantially safer formulations.58–60
Excluding HMWID, the risk of severe reactions is very low (1:250,000 administrations).57,63 Nevertheless, it is essential to administer IV iron in a setting where such adverse effects can be adequately managed.7 As with oral iron, follow up is essential.44 The suggested management pathway for IDWA is summarised in Fig 4.
Fig 4.
Flowchart summarising the management of iron deficiency without anaemia.15,49,52–55,58
Conclusion
Despite the simplicity of pathogenesis and treatment, IDWA often falls outside the scope of clinical suspicion. It is important to not only spread awareness about the condition in the medical community, but to also develop guidelines and pathways to assist clinicians. Doing so requires greater collaboration between specialists and general practitioners to identify and treat IDWA, especially preoperatively and in the context of pregnancy. In this paper, we have attempted to develop a concise guide for clinicians. Nonetheless, further research is needed to identify the best diagnostic serum markers, treatment targets, and optimal oral iron dosing regimens for IDWA.
Key points
Within the body, iron has roles other than haemoglobin synthesis.
Iron deficiency (ID) and anaemia are not synonymous; patients with ID can present with symptoms without having anaemia. Therefore iron deficiency without anaemia (IDWA) must be recognised as a clinical diagnosis on its own.
Haemoglobin ranges are based on averages and should not be used as strict cut-offs. Haemoglobin levels in the low normal range may not be normal for some people as they may be accustomed to higher levels.
Diagnosis currently depends on haemoglobin levels, ferritin levels and transferrin saturation. However, further research into the use of newer biomarkers is needed.
Many causes of ID are overlooked and clinicians need to maintain high clinical suspicion.
Females with IDWA must be treated before the start of their pregnancy to avoid the risks of perinatal and postnatal ID or IDA. These include permanent neurocognitive effects on children and having a child with a low birth weight.
ID with or without anaemia should be ruled out in the preoperative setting as failure to do so may have a negative effect on patient outcomes.
Preoperative IDWA must be studied in more depth, as the literature on this topic is sparse. There is a need for more universal pre-operative screening and treatment.
IDWA should be treated when identified. Specific guidelines on whom to treat and how to treat must be developed.
Oral iron is the first line treatment for IDWA.
Newer formulations of IV iron are much safer than traditionally thought, and should be used where oral iron is poorly tolerated or ineffective.