The following are excerpts from (1) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. [PubMed Abstract] [Full Text HTML] [Download Full Text PDF from HTML link]. Circulation. 2004 Oct 26;110(17):2747-71:
text
Echocardiography
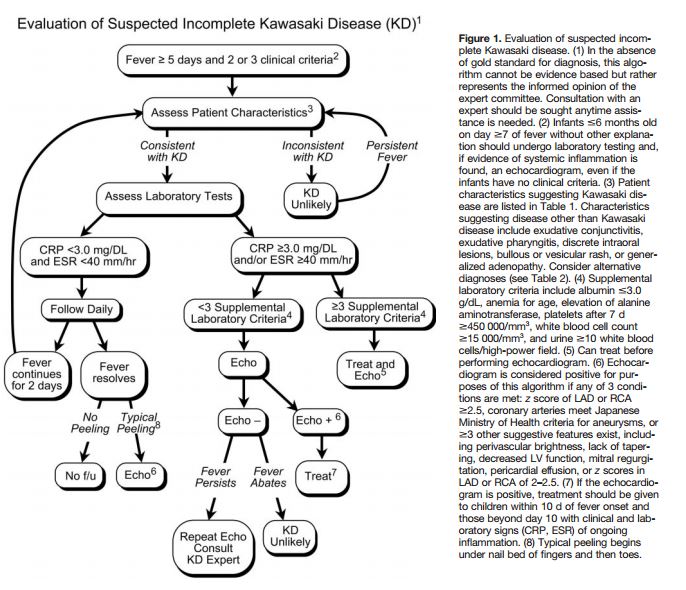
The major sequelae of Kawasaki disease are related to the cardiovascular and, more specifically, the coronary arterial system, so cardiac imaging is a critical part of the evaluation of all patients with suspected Kawasaki disease. Because it is noninvasive and has a high sensitivity and specificity for the detection of abnormalities of the proximal LMCA and RCA, echocardiography is the ideal imaging modality for cardiac assessment (evidence level C). Evaluation of the cardiovascular sequelae of Kawasaki disease requires serial cardiac ultrasound studies and should be performed using equipment with appropriate transducers and supervised by an experienced echocardiographer. The initial echocardiogram should be performed as soon as the diagnosis is suspected, but initiation of treatment should not be delayed by the timing of the study (ie, waiting for sedation). This initial study establishes a baseline for longitudinal follow-up of coronary artery morphology, LV and left valvular function, and the evolution and resolution of pericardial effusion when present. Because detailed echocardiographic imaging is compromised if a child is uncooperative, sedation often is required for younger children [the text recommends chloral hydrate which is clearly inappropriate at this time. The article was written in 2004].
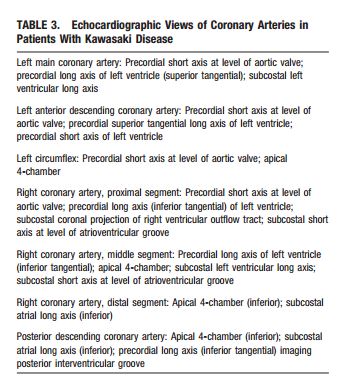
The 2D imaging should be performed with the highest frequency transducer possible. Imaging with high-frequency transducers should be attempted even in older children, as these probes allow for higher-resolution, detailed evaluation of the coronary arteries. Studies should be recorded in a dynamic video or digital cine format because the normal translational movement of the heart facilitates the display of the coronary artery anatomy. Such recordings will allow future review and comparison with subsequent studies. In addition to standard imaging from parasternal, apical, subcostal, and suprasternal notch windows, 2DE evaluation of patients with suspected Kawasaki disease should focus on imaging the LMCA, LAD, left circumflex coronary artery (LCX), RCA (proximal, middle, and distal segments), and posterior descending coronary arteries. Multiple imaging planes and transducer positions are required for the optimal visualization of all major coronary segments (Table 3, Figure 2). Maximal efforts should be made to visualize all major coronary segments. In order of highest to lowest frequency, common sites of coronary aneurysms include the proximal LAD and proximal RCA, followed by the LMCA, then LCX, and finally the distal RCA and the junction between the RCA and posterior descending coronary artery.
Evaluation of the coronary arteries should include quantitative assessment of the internal vessel diameters. Measurements should be made from inner edge to inner edge and should exclude points of branching, which may have normal focal dilation. The number and location of aneurysms and the presence or absence of intraluminal thrombi also should be assessed. Aneurysms are classified as saccular if axial and lateral diameters are nearly equal or as fusiform if symmetric dilatation with gradual proximal and distal tapering is seen. When a coronary artery is larger than normal (dilated) without a segmental aneurysm, the vessel is considered ectatic. Care must be taken in making the diagnosis of ectasia because of considerable normal variation in coronary artery distribution and dominance.
For uncomplicated cases, echocardiographic evaluation should be performed at the time of diagnosis, at 2 weeks, and at 6 to 8 weeks after onset of the disease. More frequent echocardiographic evaluation is needed to guide management in children at higher risk (eg, those who are persistently febrile or who exhibit coronary abnormalities, ventricular dysfunction, pericardial effusion, or valvular regurgitation).
Reference (2) in Discussion, p 3+4, has a well-done review of imaging including CTA and MRA as well as SPECT stress imaging. And the article points out that modern CT scanners and techniques have markedly reduced radiation exposure. SPECT scanning can also be done with markedly less radiation than in the past. See the article for details.
Resources
(1) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. [PubMed Abstract] [Full Text HTML] [Download Full Text PDF from HTML link]. Circulation. 2004 Oct 26;110(17):2747-71.
(2) Multimodality Cardiac Imaging in a Patient with Kawasaki Disease and Giant Aneurysms. [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Case Rep Pediatr. 2016;2016:4298098. Epub 2016 Oct 31.