Today, I review, link to, and excerpt from the European Society of Cardiology‘s Advanced heart failure: guideline‐directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Fail. 2022 Jun; 9(3): 1507–1523. Published online 2022 Mar 30. doi: 10.1002/ehf2.13859. [PubMed Abstract] [Full-Text HTML] [Full-Text PDF].
All that follows is from the above resource.
Abstract
Heart failure (HF) is a major cause of mortality, hospitalizations, and reduced quality of life and a major burden for the healthcare system. The number of patients that progress to an advanced stage of HF is growing. Only a limited proportion of these patients can undergo heart transplantation or mechanical circulatory support. The purpose of this review is to summarize medical management of patients with advanced HF. First, evidence-based oral treatment must be implemented although it is often not tolerated. New therapeutic options may soon become possible for these patients. The second goal is to lessen the symptomatic burden through both decongestion and haemodynamic improvement. Some new treatments acting on cardiac function may fulfil both these needs. Inotropic agents acting through an increase in intracellular calcium have often increased risk of death. However, in the recent Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF) trial, omecamtiv mecarbil was safe and effective in the reduction of the primary outcome of cardiovascular death or HF event compared with placebo (hazard ratio, 0.92; 95% confidence interval, 0.86-0.99; P = 0.03) and its effects were larger in those patients with more severe left ventricular dysfunction. Patients with severe HF who received omecamtiv mecarbil experienced a significant treatment benefit, whereas patients without severe HF did not (P = 0.005 for interaction). Lastly, clinicians should take care of the end of life with an appropriate multidisciplinary approach. Medical treatment of advanced HF therefore remains a major challenge and a wide open area for further research.
Keywords: Advanced heart failure; Diuretic therapy; Heart failure with reduced ejection fraction; Inotropes; Medical management; Omecamtiv mecarbil; Palliative care.
© 2022 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Introduction
Heart failure (HF) is a major cause of mortality, hospitalizations, and reduced quality of life and a major burden for the healthcare system. The increasing prevalence and the improved survival of HF, as well as the ageing of the population, have led to an increase of the number of patients that progress to an advanced stage of HF. 1 This poses a challenge to treating clinicians, as such patients usually experience severe symptoms and markedly impaired quality of life, become less responsive or cannot tolerate evidence‐based therapies, and are at high risk of short‐term hospitalizations and death. 2 Outcomes remain poor in patients not suitable for long‐term mechanical circulatory support (MCS) or heart transplantation 3 ; however, only a limited proportion of advanced HF patients need to be selected for advanced therapies. 2 , 4
The aim of the present review is to describe the medical management of patients with advanced HF, focusing on those with reduced ejection fraction (HFrEF) (Figure 1). Guideline‐directed medical therapy (GDMT) remains effective in patients with advanced HF. However, patients with advanced HF are less likely to tolerate it because of hypotension, low cardiac output, and severe kidney dysfunction. Physicians should be aware that the proper use of GDMT is associated with a better prognosis and its implementation is of central importance. Furthermore, new therapeutic options that may allow symptoms’ improvement and a better clinical course of HF are now available, representing a potential for further research.
Definition and epidemiology
Advanced HF can be defined as a clinical syndrome characterized by persistence of severe signs and symptoms of HF, despite optimal evidence‐based treatment. It represents the stage of the syndrome when conventional therapies are no longer effective or insufficient to control patients’ symptoms, requiring advanced therapeutic strategies including heart transplantation, MCS implantation, intermittent inotropes, and, sometimes, end‐of‐life (EOL) cares. 2 ‘Refractory’ HF may also be used as an interchangeable term although it implies a lack of response to treatment and a lack of reversibility of the impaired cardiac function and haemodynamic impairment and these conditions are not necessarily mandatory for advanced HF. Several classification systems could be applied to define patients with advanced HF, including New York Heart Association (NYHA) functional class IV referring to patients with symptoms at rest, or American College of Cardiology (ACC)/American Heart Association (AHA) stage D referring to patients who have refractory symptoms despite optimal medical therapy and require specialized interventions. 1 , 5 The first position statement defining advanced HF was published in 2007 by the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) and a more updated version was published in 2018, providing new criteria for defining advanced HF. 2 , 6 In the most recently published ESC guidelines, advanced HF has been defined as the presence of all the following criteria: (i) severe and persistent symptoms of HF [NYHA class III (advanced) or IV]; (ii) severe cardiac dysfunction [left ventricular ejection fraction (LVEF) ≤ 30% in the setting of HFrEF]; (iii) episodes of pulmonary or systemic congestion requiring high‐dose intravenous diuretics (or diuretic combinations) or episodes of low output requiring inotropes or vasoactive drugs or malignant arrhythmias causing >1 unplanned visit or hospitalization in the last 12 months; and (iv) severe impairment of exercise capacity with inability to exercise or low 6 min walking test distance (<300 m) or pVO2 < 12 mL/kg/min or <50% predicted value, estimated to be of cardiac origin. 7
Epidemiological data are still scarce, although it is estimated that 1–10% of the HF population has advanced HF. 2 In a study conducted in Minnesota, among a random sample of Olmsted County residents aged ≥45 years old, the prevalence of advanced HF (stage D according to the ACC/AHA HF staging criteria) was 0.2% of the overall population, corresponding to 10% of the HF population. 8 , 9 Most importantly, advanced HF patients are burdened with a dramatic reduction in survival. In the same cohort of patients with stage D HF, mortality at 5 year was 80%. 10 In a more recent study, of 6836 adults with HF, 936 (13.7%) met ESC diagnostic criteria for advanced HF. 9 The median (interquartile range) time from advanced HF diagnosis to death was 12.2 months (3.7–29.9 months). 9 Similarly, other studies reported high mortality rates. In the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial, enrolling end‐stage HF patients ineligible for heart transplantation, the rates of mortality were 75% at 1 year and 92% at 2 years in the medical therapy group [vs. 48% and 77%, respectively, in those receiving left ventricular assist device (LVAD)]. 11 Of note, the ineligibility to heart transplantation might have selected a population at higher risk.
Treatment to improve outcome
Evidence‐based therapy for heart failure with reduced ejection fraction
Neurohormonal antagonists, including angiotensin‐converting enzyme inhibitors (ACEi), angiotensin receptor blockers, angiotensin receptor neprilysin inhibitors, beta‐blockers, and mineralocorticoid receptor antagonists, and sodium‐glucose co‐transporter 2 (SGLT2) inhibitors, are the mainstay of HFrEF treatment, improving the clinical course of HF. 7 , 12 , 13 , 14 , 15 , 16 Adherence to GDMT is associated with improved outcome. 17 , 18 , 19 , 20 , 21 , 22 Data from population‐based studies reported a decline in HF‐related hospitalizations and mortality over the last two decades. However, no further improvement was reported in the most recent years because of the lack of positive trials until 2019. 16 , 23 Implementation of GDMT remains a cornerstone of treatment of also the patients with advanced HF and reduced LVEF. Indeed, many trials enrolling patients with severe HF, NYHA class III–IV, and severely impaired LVEF, namely, Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II), Carvedilol Prospective Randomized Cumulative Survival Study (COPERNICUS), and the Randomized Aldactone Evaluation Study (RALES), consistently showed clinical benefits of these drugs among a population with a more advanced stage of the disease. 24 , 25 , 26 , 27 However, patients with advanced HF often do not tolerate neurohormonal modulators because of hypotension, low cardiac output, and severe kidney dysfunction. The development of circulatory haemodynamic limitations to ACEi identifies patients with severe HF and with mortality over 50% at 1 year. 28 Recently, new drugs have demonstrated benefits in patients with HFrEF, namely, SGLT2 inhibitors, vericiguat and omecamtiv mecarbil. These drugs may be more tolerated as they do not decrease systolic blood pressure meaningfully and have neutral or favourable (SGLT2 inhibitors) long‐term effects on the progression of kidney dysfunction. 29
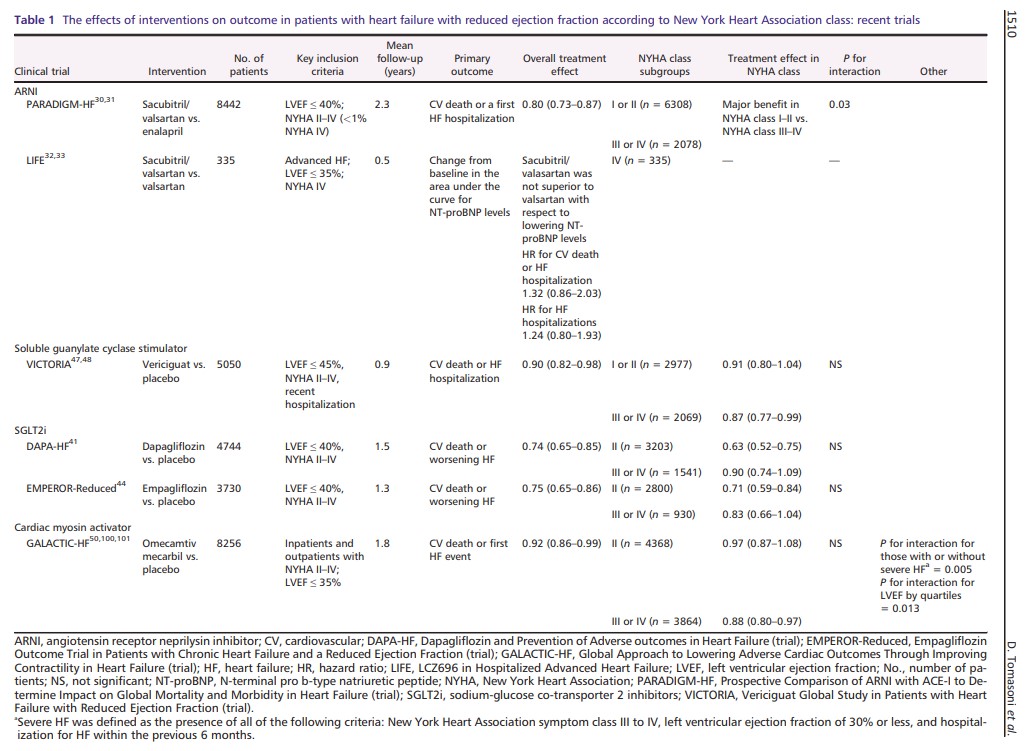
Table 1 shows the proportion of patients with advanced HF enrolled in the most recent trials and treatment interaction. In the Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor with ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial, <1% of patients had an NYHA functional class IV. Pre‐specified subgroup analyses showed a significant interaction between NYHA class at randomization and the effect of treatment on the primary endpoint, with major benefits in the subgroup of patients with NYHA class I or II vs. NYHA class III or IV. 30 , 31 This interaction was not observed considering cardiovascular (CV) death. Thus, the benefit of sacubitril/valsartan in more severe patients remained uncertain. The rationale of the LIFE (LCZ696 in Hospitalized Advanced Heart Failure) study was to assess the feasibility, efficacy, and safety of such treatment in the most advanced phases of the disease. This 24 week prospective, multicentre trial compared the use of sacubitril/valsartan vs. valsartan alone in NYHA class IV patients with an LVEF ≤ 35% and elevated levels of N‐terminal pro b‐type natriuretic peptide (NT‐proBNP). The primary endpoint was the proportional change from baseline in the area under the curve for NT‐proBNP levels, while secondary and tertiary endpoints consisted of an assessment of clinical outcome, safety, and tolerability. 32 The study was prematurely stopped due to the Coronavirus Disease 2019 (COVID‐19) pandemic, but 335 patients were enrolled and results have been recently presented. 33 Neither treatment with sacubitril/valsartan nor valsartan decreased the median NT‐proBNP levels below baseline through 24 weeks. Sacubitril/valsartan did not improve the clinical composite of number of days alive, out of hospital, and free from HF events and did not decrease the risk of death from CV causes or HF hospitalization, nor all‐cause death, compared with valsartan. Results may be influenced by the sample size and the study duration, which was shorter than previous studies. Furthermore, the study was not powered to detect changes in CV death and/or HF hospitalizations. Importantly, there was no safety concern even if 72 eligible patients (18%) were not able to tolerate sacubitril/valsartan during the short run‐in period, and 49 patients (29%) discontinued sacubitril/valsartan during the 24 weeks of the trial. A recent real‐life study investigated the administration of sacubitril/valsartan in a real‐world cohort of more advanced HFrEF patients, with a worse clinical status than those enrolled in the PARADIGM‐HF trial. During the 6 month follow‐up, the rates of hospitalizations, NT‐proBNP levels, and the need for ambulatory levosimendan decreased and a reverse cardiac remodelling was observed in patients treated with sacubitril/valsartan. No major adverse effects were reported. 34 Martens et al. showed that patients receiving sacubitril/valsartan in clinical practice, compared with those in PARADIGM‐HF, were burdened by more severe disease. In this advanced population, sacubitril/valsartan significantly improved the NYHA class, despite a higher risk of systolic blood pressure drop compared with that reported in PARADIGM‐HF. 35 In the most recently published ESC guideline, sacubitril/valsartan (if tolerated) is recommended as a replacement for an ACEi in patients with HFrEF to reduce the risk of HF hospitalization and death. 7 On the other hand, patients with advanced HF may become intolerant to sacubitril/valsartan and this may be a reason of de‐escalation.
Sodium‐glucose co‐transporter 2 inhibitors act on new therapeutic pathways, different from those on which neurohormonal agents are active. 36 , 37 , 38 , 39 Beyond the diuretic and haemodynamic effects, SGLT2 inhibitors could also have an impact on myocardial metabolism, ion transporters, fibrosis, adipokines, and vascular function. 40 In the DAPA‐HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial, dapagliflozin reduced the risk of the primary composite endpoint of CV death or worsening HF, compared with placebo, in patients with HFrEF, regardless of diabetes’ history [hazard ratio (HR), 0.74; 95% confidence interval (CI), 0.65–0.85; P < 0.001]. 41 Dapagliflozin also improved physical function and the quality of life, measured through Kansas City Cardiomyopathy Questionnaire (KCCQ)*. 42
*See Links To Resources On The Kansas City Cardiomyopathy Questionnaires
Posted on February 11, 2024 by Tom Wade MD.
Importantly, dapagliflozin was safe and well tolerated, even in patients with a baseline systolic blood pressure < 110 mmHg, and the absolute benefit of the drug was large in those with the lowest systolic blood pressure, opening future perspective for the treatment of advanced HFrEF patients. 43 The more recent Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR‐Reduced) trial confirmed and extended the benefits of SGLT2 inhibitors in stable, more advanced HF population, with a reduction in the risk of CV death or HF hospitalization compared with placebo (HR, 0.75; 95% CI, 0.65–0.86; P < 0.001). 44 SGLT2 inhibitors also showed a slower decline in the estimated glomerular filtration rate. Given their early benefits, safety profile, and tolerability, an early upfront initiation of SGLT2 inhibitors has been supported by HF experts. 45 Adverse effects usually associated with the use of neurohormonal antagonist (hypotension, bradycardia, and hyperkalaemia) were not described with SGLT2 inhibitors, an aspect that may represent a particular advantage of SGLT2 inhibitors among fragile patients with advanced HF.
Advanced symptomatic HFrEF patients who had recently been hospitalized or had received intravenous diuretic therapy were enrolled in the Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) trial. The recruitment of sicker patients with higher NT‐proBNP levels than those in previous HF trials was a purpose of the study and resulted in a higher risk of events. 46 The MAGGIC (Meta‐Analysis Global Group in Chronic Heart Failure) risk score in VICTORIA was higher as compared with the MAGGIC risk score in PARADIGM‐HF. Similarly, the proportion of NYHA class III or IV patients was 41% in the VICTORIA trial, compared with 25% in the PARADIGM‐HF and EMPEROR‐Reduced and 32% in the DAPA‐HF. 46 In this high‐risk population, the novel oral soluble guanylate cyclase stimulator, vericiguat, reduced the composite endpoint of CV death or HF hospitalization compared with placebo (HR, 0.90; 95% CI, 0.82–0.98; P = 0.02). 47 However, subgroup analysis showed an interaction between treatment and the primary outcome according to pre‐specified quartiles of NT‐proBNP with the benefits of vericiguat shown only in patients with NT‐proBNP levels up to 8000 pg/mL. 48 Patients who may benefit from vericiguat should be better defined, especially in the light of a possible individualized approach. 49
The positive results of the GALACTIC‐HF (Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure) trial, comparing omecamtiv mecarbil with placebo, will be discussed in the following chapter on inotropes. 50 Importantly, this is the first inotrope that showed benefits on clinical outcome in patients with chronic HF.
Treatment to improve symptoms
Management of congestion
Each HF‐related hospitalization increases the risk for subsequent events. Worsening of congestion, with symptoms and signs of fluid overload and/or fluid redistribution, remains the major cause of hospitalization for acute HF or unplanned visits requiring intravenous diuretic treatment and this is increasingly frequent in advanced stages. 51 Moreover, persistently elevated left ventricular filling pressure is common in advanced HF and has prognostic significance. 52 Remote monitoring of congestion and pulmonary artery pressure‐guided pharmacotherapy may represent a helpful tool to reduce HF hospitalization in outpatients with advanced HF. 53 , 54 , 55 , 56 Assessment and management of congestion in patients with advanced HF has been recently reviewed. 57
The standard treatment for congestion is represented by loop diuretics, with furosemide being first choice. 58 , 59 However, in patients with advanced HF, the management of congestion can sometimes be difficult due to the high prevalence of cardiorenal syndrome. Chronically decreased perfusion and venous congestion compromise renal function. 60 Prolonged diuretic treatment leads to nephron remodelling, one of the main mechanisms behind diuretic resistance: hypertrophy and hyperplasia of the distal convoluted tubule cells, principal cells, and intercalated cells generates a gain of function, with an increased reabsorption capacity of the distal nephron. 61 When diuresis is insufficient, uptitration of oral loop diuretics should represent the first therapeutic option. Planned ambulatory intravenous administration of loop diuretics may help maintaining fluid balance and, in cases of inadequate responses, home administration of intravenous loop diuretics is suggested. Thiazide‐like drugs or metolazone are commonly used as an adjuvant therapy, along with loop diuretics, both in refractory outpatients and in acute decompensated HF. 58 , 62 However, evidence is still limited and the risk of worsening renal function or electrolyte disorders, namely, hypokalaemia and hyponatraemia, must be considered. In a propensity analysis of 13 898 hospitalized patients with acute HF, metolazone was associated with increased mortality (adjusted HR, 1.20; 95% CI, 1.04–1.39; P = 0.01). 63 As advanced stages of HF are characterized by inappropriately high levels of arginine vasopressin, leading to plasma expansion and dilutional hyponatraemia, the selective V2 receptor antagonist tolvaptan may be considered as a further decongestive strategy. In pre‐clinical HF models, the novel dual acting vasopressin V1a/V2 receptor antagonist pecavaptan showed a better haemodynamic effect compared with tolvaptan, including increase in cardiac output and cardiac index and decrease in total peripheral resistance, and the first clinical results will be available soon. 64 , 65
When the previous therapies have failed, ultrafiltration (UF) should be considered. 66 Despite safety issues, UF is associated with greater weight reduction and volume depletion and with shorter hospitalizations when patients are admitted to the hospital. UF rates superior to 250 mL/h are not recommended, and patients with right HF could only take lower rates. 67 Haematocrit and patient’s weight must be closely monitored during the UF, so that treatment can be eventually stopped and resumed safely. Finally, peritoneal dialysis might be an at‐home option for patients not responding to conventional diuretic therapy. It offers many advantages, including a preserved renal function, haemodynamic stability, and less inflammation compared with haemodialysis. This strategy can lead to weight loss and a better NYHA classification and quality of life, reducing the length of in‐hospital stay. 68 However, further large, controlled, randomized studies are needed to better evaluate and define this strategy. 2
Inotropic agents: rationale and classification
Patients with end‐stage HF, who are otherwise in good health, without significant non‐cardiac comorbidities, should be referred for heart transplantation. Heart transplantation represents the gold standard treatment in such patients, with 1 year survival of almost 90% and a median survival of 12.5 years. 69 Nonetheless, it represents a limited therapeutic option, due to the disproportion between donors and possible candidates needing the transplant. Long‐term MCS is a valid alternative in patients non‐eligible to heart transplantation or in those deteriorating while waiting for transplantation. 11 MCS implantation is burdened by high costs and adverse events, limiting its use and requiring restrictive clinical criteria as well. 1 Thus, in patients with low cardiac output with end‐organ hypoperfusion, ineligible to heart transplantation or LVAD implantation, inotropes may represent a rescue strategy to improve haemodynamics. Inotrope use aims to maintain an adequate cardiac output and reduce filling pressures by enhancing cardiac contractility and, for some inotropes, also by vasodilatation and may represent a potentially useful strategy also in the chronic treatment of advanced HF, besides their role as short‐term therapies.
Psotka et al. have recently classified inotropes in calcitropes, which modulate calcium signalling, myotropes, acting on the sarcomere through a calcium‐independent mechanism, and mitotropes, which exert their action on mitochondrial energy production. 70 Calcitropes include the traditional inotropes: catecholamines, phosphodiesterase (PDE)‐3 inhibitors, and cardiac glycoside (i.e. digitalis). Among catecholamines, which act on the β‐adrenoceptor‐adenylyl cyclase system, epinephrine and norepinephrine are vasopressors mainly used in an acute cardiogenic shock, as a bridge to haemodynamic stability. 71 Dobutamine mainly acts on β1 cardiac receptors rather than α1 and β2 vascular receptors, increasing stroke volume and without causing peripheral vasoconstriction. PDE‐3 inhibitors, including amrinone, milrinone, and enoximone, inhibit the enzyme PDE‐3 with consequently increased concentrations of available cAMP and intracellular calcium. Milrinone is currently the most widely used drug within this class, followed by enoximone. Both drugs were associated with an improvement in haemodynamics and functional capacity in patients with advanced HF. 72 Levosimendan is a PDE inhibitor with additive properties, making it able to increase calcium sensitivity during systole without impairing diastolic relaxation. This action leads to increased cardiac output, reduced wedge pressures, peripheral vasodilation, and symptoms relief. 73 , 74 Istaroxime exerts a dual function: on one hand, it stimulates sarcoplasmatic reticulum Ca2+‐ATPase SERCA2a; on the other hand, it inhibits the Na‐K pump, resulting in both an inotropic and a lusitropic effect. 75 Omecamtiv mecarbil—the first drug of the myotrope class—is a direct activator of cardiac myosin in a calcium‐independent manner. It increases the contractile force by enforcing the interaction between myosin and actin. 76 , 77 Results from non‐clinical studies and a randomized, phase 2a trial investigating another cardiac myosin activator, danicamtiv, were recently published. 78 Mitotropes are currently under study in HFrEF patients, with promising results in small clinical studies, but there is a lack of randomized trials providing more consistent results. 70
Inotropic agents: negative results from randomized controlled clinical trials
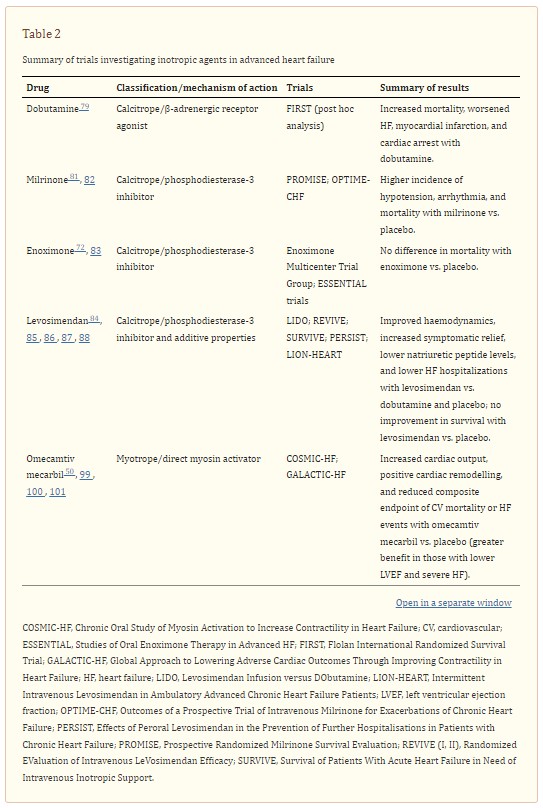
Despite its theoretical potential benefit among patients with worsening, advanced HF, there is a lack of dedicated studies evaluating dobutamine in this particular setting. In a post hoc analysis of the Flolan International Randomized Survival Trial (FIRST), a study investigating the use of epoprosteonol, treatment with intravenous continuous dobutamine was associated with higher 6 month mortality rate in patients with advanced HF (70.5% vs. 37.1% in controls; P < 0.001). Also, the occurrence of first event, including worsening HF, need for vasoactive medications, resuscitated cardiac arrest, and myocardial infarction, was higher in the dobutamine group. 79 Although results were confirmed after adjustment for baseline characteristics, the study was not designed to compare dobutamine with placebo and the selection of sicker patients requiring inotropes might have influenced the results. In a retrospective single‐centre study, continuous intravenous home dobutamine was associated with improvement in symptomatic status and HF hospitalizations in 21 end‐stage HF patients. 80
Among 1088 ambulatory patients with severe chronic HF, oral milrinone was associated with an increase in all‐cause and CV mortality and this effect was also more evident in those with the most severe symptoms (NYHA IV). 81 In the OPTIME‐CHF (Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure) trial, 951 patients admitted with an exacerbation of chronic HF not requiring intravenous inotropic support were randomized to receive a 48 h infusion of either milrinone (0.5 μg/kg/min) or saline placebo. Results showed no difference in incidence of death or readmission; however, milrinone was associated with a higher incidence of sustained hypotension requiring intervention (10.7% vs. 3.2% in the placebo group; P < .001) and new atrial arrhythmias (4.6% vs. 1.5%; P = 0.004). 82 Similarly, enoximone did not improve survival in the Studies of Oral Enoximone Therapy in Advanced HF (ESSENTIAL) programme. 83
Levosimendan Infusion versus DObutamine (LIDO) Study showed that levosimendan improved haemodynamic performance more effectively than dobutamine in patients with severe, low‐output HF. 84 The Randomized EValuation of Intravenous LeVosimendan Efficacy (REVIVE) and Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trials examined safety and efficacy of levosimendan in patients with acute decompensated HF, compared with placebo and dobutamine, respectively. 85 , 86 In the SURVIVE trial, 180 day mortality (the primary endpoint) was not different between dobutamine and levosimendan. 86 In the REVIVE trial, levosimendan was associated with more frequent hypotension and cardiac arrhythmias during the infusion period compared with placebo, and a non‐significant risk of death. 85 Both trials showed benefits in terms of symptomatic relief and decrease in natriuretic peptide levels. The PERSIST trial (Effects of Peroral Levosimendan in the Prevention of Further Hospitalisations in Patients with Chronic Heart Failure) firstly showed improvement in quality of life and decrease in NT‐proBNP levels in patients with severe chronic HF (NYHA class IIIB–IV and LVEF < 30%) treated with oral levosimendan compared with placebo. 87 More recently, in the small, multicentre, randomized, placebo‐controlled LION‐HEART trial (Intermittent Intravenous Levosimendan in Ambulatory Advanced Chronic Heart Failure Patients), intermittent levosimendan decreased NT‐proBNP levels and reduced HF rehospitalizations (HR, 0.25; 95% CI, 0.11–0.56; P = 0.001). 88 Similar results were reported in other small studies. 89 , 90 , 91 , 92 The ongoing LeoDOR study (NCT03437226) will assess the efficacy and safety of repetitive levosimendan given for 12 weeks in advanced HF patients. 93
Randomized controlled trials with drugs acting through an increase in intracellular calcium failed to prove benefits in terms of outcome, with an increase in mortality in some cases. The reasons behind this failure may be multiple. 94 First, haemodynamic improvement and symptomatic relief do not necessarily translate into an improvement in outcome. 95 Increasing contractility in a failing heart may induce short‐term benefits in terms of symptoms and also increase myocardial work and oxygen consumption with long‐term deterioration of myocardial function. Second, benefits of chronic inotropic use might be limited to specific HF phenotypes (i.e. ischaemic vs. non‐ischaemic). 96 Third, the effects may be dose dependent and mainly due to an increase in sudden death so that administration of lower doses and concomitant beta‐blocker and implantable cardioverter defibrillator treatment may prevent untoward effects. 83 , 94 , 97
A recent meta‐analysis including 66 studies showed that in patients receiving ambulatory inotrope infusions, there was a greater improvement in NYHA functional class than in controls, without a significant effect on mortality risk (pooled risk ratio, 0.68; 95% CI, 0.40–1.17; P = 0.16; 9 trials). 98 Improvement in quality of life and functional capacity, with neutral impact on survival, should represent the ideal target of chronic inotropic use in the setting of advanced HF. Ahmad et al. suggest that, besides a careful selection of patients with advanced HF (not still in an early phase of the disease but also not too advanced), future trials testing inotropes should include patients who are already on maximally tolerated medical therapy (including beta‐blockers) and already have received implantable cardioverter defibrillator (if indicated). Cardiorenal biomarkers may be used with safety purpose and a run‐in phase might be useful to exclude patients with cardiac injury or severe adverse effects. 94