In this post, I link to and excerpt from Diagnosis and Treatment for Mild Cognitive Impairment: A Systematic Review of Clinical Practice Guidelines and Consensus Statements [Abstract] [Full-Text HTML] [Full-Text PDF]. Front Neurol. 2021 Oct 12;12:719849. doi: 10.3389/fneur.2021.719849. eCollection 2021.
All that follows is from the above resource. This is an excellent article worth reading in its entirety. The guidelines and consensus statements reviewed have differing recommendations.
Abstract
Background: Mild cognitive impairment (MCI) is an important stage between the normal cognitive decline of aging and dementia. The aim of this study was to compare and harmonize the recommendations for the diagnosis and treatment of MCI based on current clinical practice guidelines. Methods: We searched the PubMed, EMBASE, China National Knowledge Infrastructure, Wanfang Database, Chinese Science and Technology Periodical Database, and Chinese Biological Medicine Database from their inception date to April 24, 2021 to identify all published guidelines on MCI. The qualities of the eligible guidelines were appraised by two reviewers using the Appraisal of Guidelines for Research and Evaluation II instrument. Results: Thirteen guidance documents (four guidelines and nine consensus statements) with specific recommendations were included. Nine guidelines and consensus statements covered the screening and diagnosis of MCI. The evaluation of the documents showed that neuropsychological testing and biomarker assessments were the most common recommendations for the diagnosis of MCI. Nine of the 13 guidance documents covered the treatment and management of MCI. The recommendations for the treatment and management were classified into four categories, namely: intervention for risk reduction, pharmacologic interventions, non-pharmacologic interventions, and counseling. Regarding pharmacological interventions, three guidelines recommend no pharmacologic intervention. The use of cholinesterase inhibitors for MCI is contraindicated in three guidance documents, whereas one proposes that cholinesterase inhibitors and memantine should be deprescribed. EHb761®, Chinese herbal decoctions, and Chinese traditional patent medicine are recommended in two documents. A total of seven guidance documents recommend non-pharmacological interventions, including physical activity interventions, cognitive interventions, dietary and nutritional interventions, and acupuncture. Conclusion: An updated search for possible evidence on the diagnosis and treatment of MCI is needed. Potentially effective diagnoses and treatments, either conventional or complementary, and alternative therapies should be highly valued and addressed in correlation with the supporting evidence.
Keywords: diagnosis; guidelines; mild cognitive impairment; systematic review; therapeutic.
Copyright © 2021 Chen, Liang, Li, Yang, Wang and Shi.
Results
Selection of Guidelines
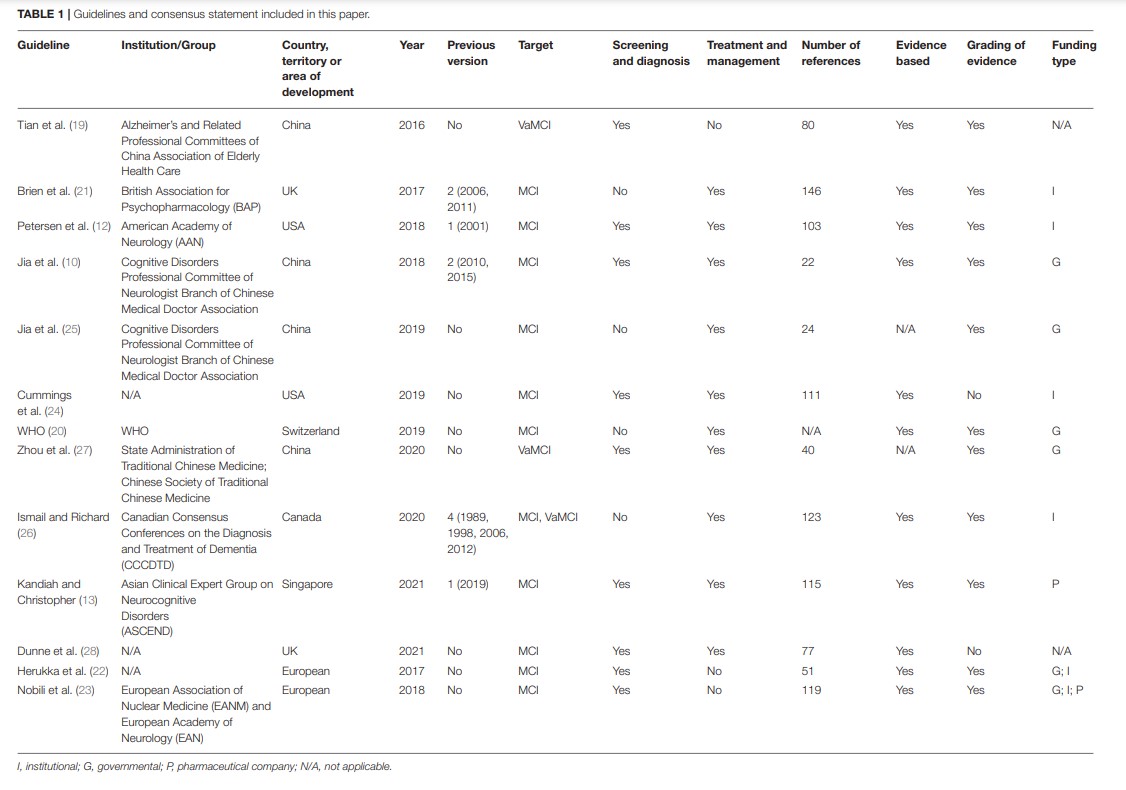
A total of 13 guidance documents with specific recommendations were eligible, including four guidelines (10, 12, 19, 20) and nine consensus statements (13, 21–28).
Two documents (12, 24) were developed in the United States, two (21, 28) in the United Kingdom, four (10, 19, 25, 27) in China, one (26) in Canada, one (13) in Singapore, one (20) in Switzerland, and two (22, 23) in Europe. Five of the documents had previous versions (10, 12, 13, 21, 26). Eleven documents (10, 12, 13, 20–26, 28) are directed at MCI populations and three documents (19, 26, 27) are directed at vascular mild cognitive impairment (VaMCI) populations. Recommendations for screening and diagnosis are included in nine documents (10, 12, 13, 19, 22–24, 26, 28), whereas recommendations for treatment and management are included in nine documents (10, 12, 13, 20, 21, 24–27). Nine documents state that the guideline/statement was developed using a systematic search strategy (10, 12, 19–24, 26). The detailed characteristics of the eligible guidelines and consensus statements are presented in Table 1.
A summary of the grading systems used in the included guidelines and consensus statements is presented in Supplementary Table 2.
Quality Assessment
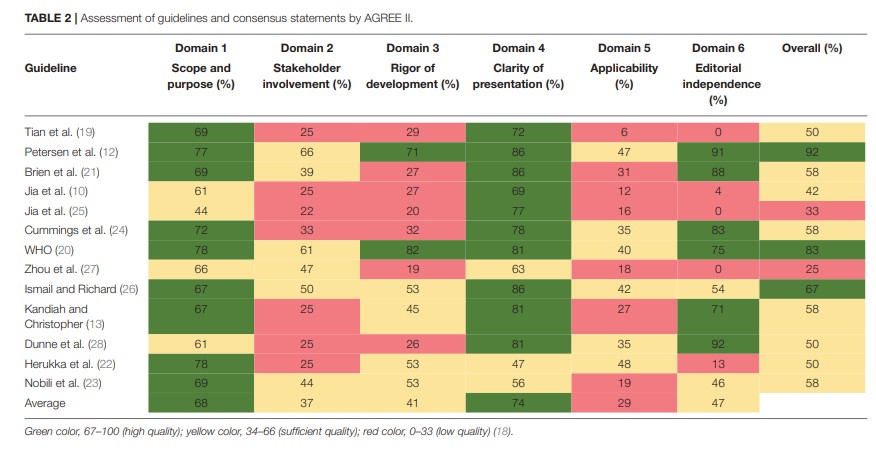
The scores for each domain of the AGREE II instrument are presented in Table 2. The guideline developed by the American Academy of Neurology (AAN) had the highest overall score in the six domains (12).
Screening and Diagnosis
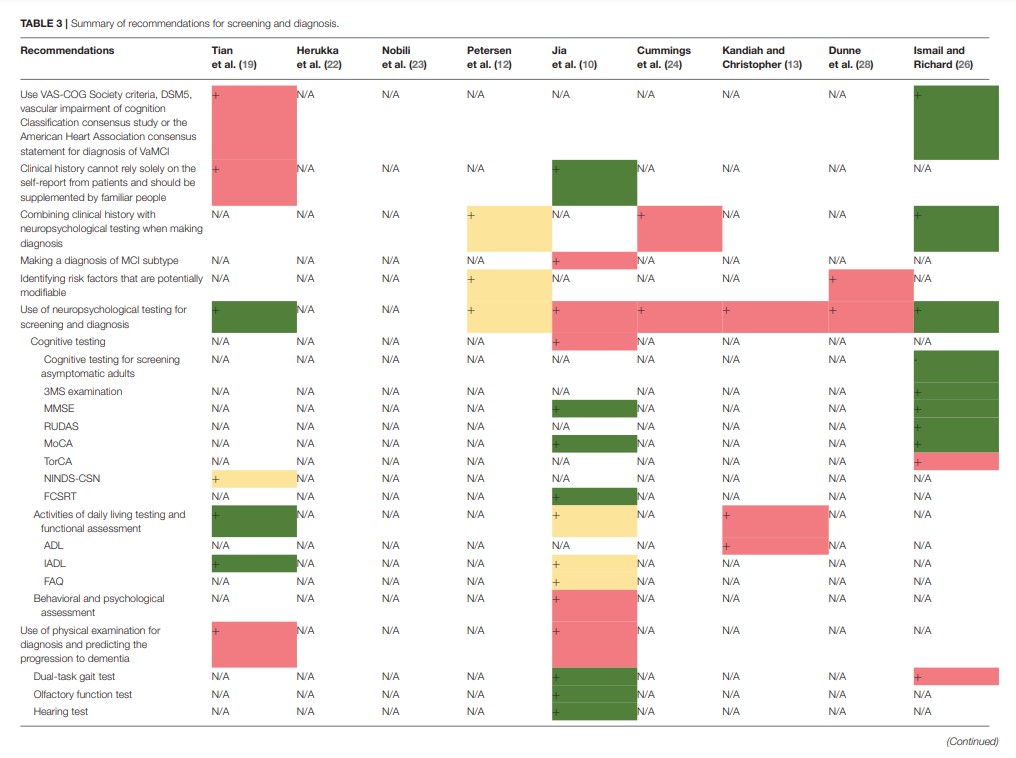
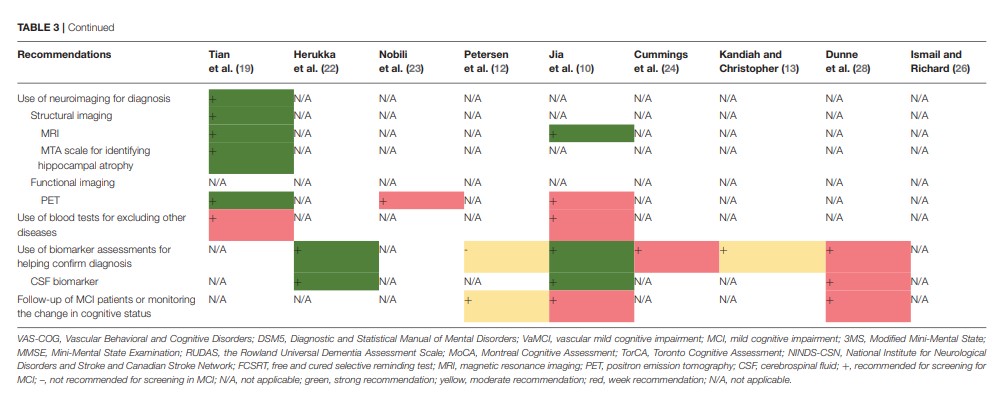
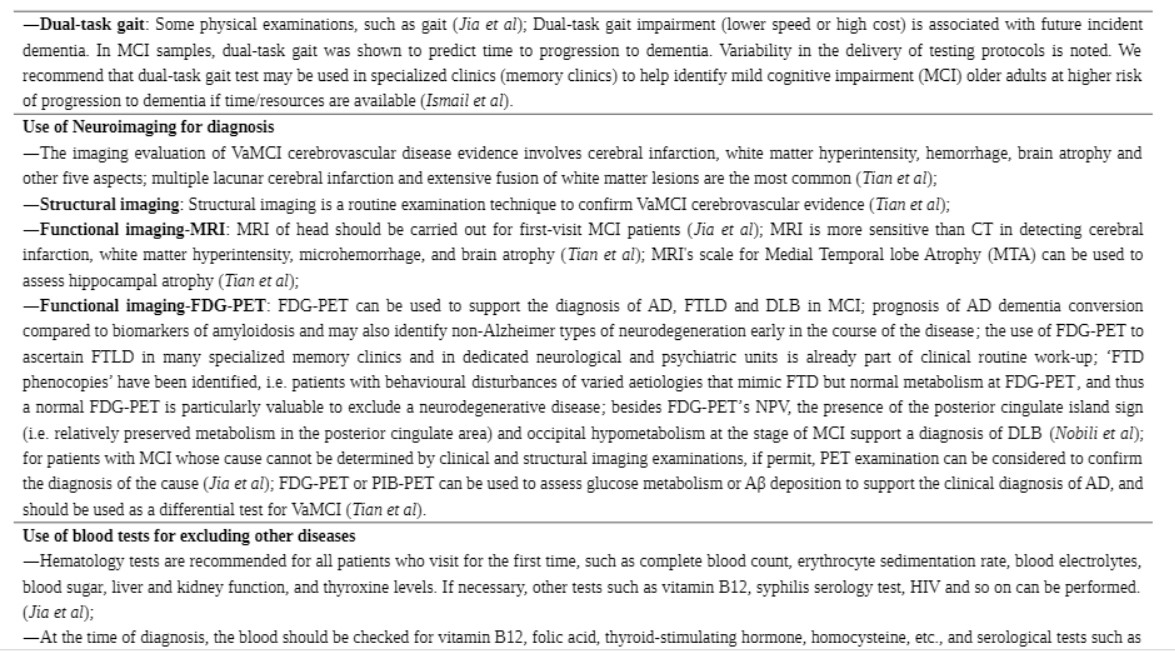
Nine guidelines and consensus statements covered the screening and diagnosis of MCI (10, 12, 13, 19, 24, 26, 28). Neuropsychological testing and biomarker assessments are the most recommended tests for the diagnosis of MCI. There was agreement between two guidance documents recommending that standard diagnostic criteria, such as the Vascular Behavioral and Cognitive Disorders Society criteria, Diagnostic and Statistical Manual of Mental Disorders 5, Vascular Impairment of Cognition Classification Consensus Study, or the American Heart Association consensus statement, should be used in the diagnostic process for VaMCI (19, 26). Two guidelines recommend that self-report from patients should not be solely relied on for clinical history but should be supplemented by reports from people familiar with the patient (10, 19). Three guidance documents indicate that clinicians should combine clinical history with neuropsychological testing in the diagnostic process (12, 24, 26). One guideline recommends making a diagnosis of MCI subtype (10). Two guidance documents recommend that clinicians identify the MCI risk factors that are potentially modifiable (12, 28). Seven documents (10, 12, 13, 19, 24, 26, 28) recommend the use of neuropsychological testing for screening and diagnosis. Three (10, 19, 26) of these seven documents recommend cognitive testing, including assessment using the Modified Mini-Mental State (3MS) examination, the MMSE, the Rowland Universal Dementia Assessment Scale (RUDAS), MoCA, Toronto Cognitive Assessment (TorCA), National Institute for Neurological Disorders and Stroke and Canadian Stroke Network (NINDS-CSN), and FCSRT; three (10, 13, 19) recommend testing activities of daily living and functional assessment, including assessment using the Activity of Daily Living Scale (ADL), Instrumental Activity Daily Living (IADL) scale, and Functional Activities Questionnaire (FAQ); and one document (10) recommends behavioral and psychological assessment. Three documents (12, 24, 28) do not recommend a specific diagnostic tool, and one guidance document does not recommend cognitive testing for screening asymptomatic adults (26). Three guidance documents (10, 19, 26) propose that a physical examination needs to be conducted for diagnosis and for the prediction of the progression to dementia. Two (10, 26) of these three documents recommend dual-task gait test, while one document recommends the olfactory function test and hearing test (10). Regarding diagnostic radiologic examinations, two documents (12, 19) recommend magnetic resonance imaging (MRI), and three documents (12, 19, 23) recommend positron emission tomography (PET). One guideline recommends the assessment of medial temporal lobe atrophy for the identification of hippocampal atrophy (19). Two guidelines recommend conducting blood tests to exclude other potential diseases (10, 19). Five guidance documents (10, 13, 22, 24, 28) recommend biomarker assessments to help confirm the diagnosis of MCI. Three (10, 22, 28) of these documents recommend cerebrospinal fluid biomarker tests (CSF tau protein and CSF β-amyloid 42), whereas another guideline (12) suggests that there is no accepted biomarker for a definite diagnosis. Three guidance documents recommend follow-up or monitoring the changes in the cognitive status of a patient with MCI (10, 12, 28). The details and levels of recommendations for screening and diagnosis are presented in Table 3 and Supplementary Table 3A.
Treatment and Management
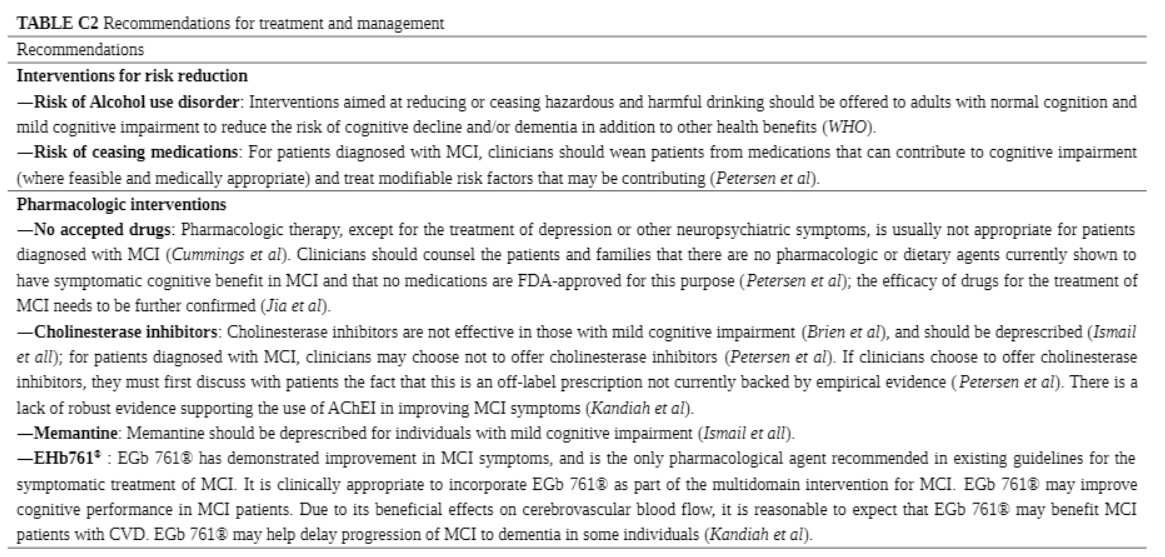
Nine guidance documents covered recommendations for the treatment (10, 12, 13, 20, 21, 24–27). The recommendations for treatment and management were classified into four categories: intervention for risk reduction, pharmacologic interventions, non-pharmacologic interventions, and counseling. Regarding intervention for risk reduction, one guideline (20) recommends that patients reduce or cease harmful drinking, whereas another guideline (12) recommends discontinuing medications that can contribute to cognitive impairment. Seven guidance documents include recommendations for pharmacologic interventions (10, 12, 13, 21, 24, 26, 27). Three guidelines (10, 12, 24) indicate that there is no accepted drug for the treatment of MCI. Three guidance documents (12, 13, 21) contraindicate cholinesterase inhibitors for the treatment of MCI, whereas one (26) proposes that cholinesterase inhibitors and memantine should be deprescribed.
Strengths and Limitations
To the best of our knowledge, this is the first paper to summarize the published guideline recommendations for MCI. The main strength of this study is the comprehensive and systematic literature search that was conducted to identify guidance documents related to the diagnosis and treatment of MCI. The guidelines and consensus statements were independently appraised by two reviewers using AGREE II, and the finding on the methodological problems, mainly in the applicability domain, presented as lacking implementation strategies and related application resources, may help in the development of future MCI guidelines. The recommendations were summarized into key recommendations shown in tables, and the consistency of the recommendations and the difference between them were compared.
The limitation of this study is that, although a systematic search strategy was implemented, certain guidelines may have been missed because of language limitations. Moreover, there were no accepted cutoff points for the domain scores of AGREE II. In addition, the fact that we referred to a previous article for the grading of the domain scores might be a matter of dispute (17, 18).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.719849/full#supplementary-material
Here is some of the supplementary material:
yellow: moderate recomendation; red: weak recomendation; N/A: not applicable