I posted on this article previously but I wanted to do redo the post, so here it is.
In addition to the resource below, please review The Pediatrics On Call Podcast, [Link to the complete list of episodes] Identifying and Treating Myocarditis, Promoting Healthy Sexuality for Youth with Disabilities – Episode 76. 08/24/2021:
In this episode Stuart Berger, MD, FAAP, chair of the executive committee of the AAP Section on Cardiology and Cardiac Surgery, describes causes, symptoms, and treatment of myocarditis. Hosts David Hill, MD, FAAP, and Joanna Parga-Belinkie, MD, FAAP, also talk to Amy Houtrow, MD, PhD, MPH, FAAP, about ways pediatricians can promote healthy sexuality for youth with disabilities.
In this post, I link to and excerpt from Diagnosis and Management of Myocarditis in Children: A Scientific Statement From the American Heart Association [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Circulation. 2021 Aug 10;144(6):e123-e135.
All that follows are excerpts from the above resource.
ABSTRACT:
Myocarditis remains a clinical challenge in pediatrics. Originally, it was recognized at autopsy before the application
of endomyocardial biopsy, which led to a histopathology-based diagnosis such as in the Dallas criteria. Given the invasive
and low-sensitivity nature of endomyocardial biopsy, its diagnostic focus shifted to a reliance on clinical suspicion. With theadvances of cardiac magnetic resonance, an examination of the whole heart in vivo has gained acceptance in the pursuit of
a diagnosis of myocarditis. The presentation may vary from minimal symptoms to heart failure, life-threatening arrhythmias, or cardiogenic shock. Outcomes span full resolution to chronic heart failure and the need for heart transplantation with inadequate clues to predict the disease trajectory.The American Heart Association commissioned this writing group to explore the current knowledge and management within the field of pediatric myocarditis. This statement highlights advances in our understanding of the immunopathogenesis, new and shifting dominant pathogeneses, modern laboratory testing, and use of mechanical circulatory support, with a special emphasis on innovations in cardiac magnetic resonance imaging. Despite these strides forward, we struggle without a universally accepted definition of myocarditis, which impedes progress in disease-targeted therapy.
Key Words: AHA Scientific Statements ◼ heart disease ◼ immune system diseases ◼ infections ◼ inflammation ◼ myocarditis
◼ pediatrics ◼ ventricular dysfunctionDefining Myocarditis
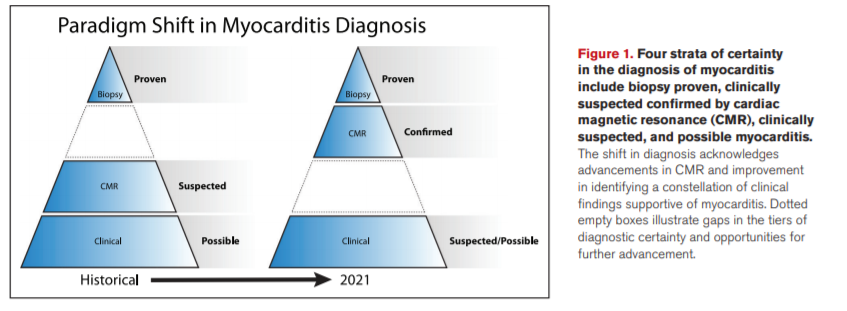
Efforts to define myocarditis have evolved. Initially, pathologic evidence of an inflammatory cardiomyopathy was required. Currently, biopsy rates are declining as practitioners increasingly rely on cardiac magnetic resonance (CMR) imaging and incorporation of clinical and laboratory criteria.1,2 Guided by review of the literature, expert opinion, and modern practice patterns, this statement recognizes 4 strata that can confirm the diagnosis: (1) biopsy proven, (2) CMR-confirmed clinically suspected, (3) clinically suspected, and (4) possible myocarditis (Figure 1). The paradigm shift in diagnosis acknowledges advancements in CMR but needs and deserves further study of its accuracy. A positive biopsy proves myocarditis, but like CMR, a negative result does not necessarily rule it out. Clinically suspected myocarditis represents a constellation of findings supportive of the diagnosis of myocarditis when biopsy and CMR cannot be performed or despite their negative results. To date, there are no definitive criteria using clinical features alone to confirm the diagnosis of myocarditis or to differentiate clinical suspicion from possible myocarditis. Nevertheless, this statement discusses clinical and laboratory features that may upgrade a case from possible to clinically suspected myocarditis in the current era. Accuracy (specificity and sensitivity) of most of the testing in a properly studied population is lacking. Hence, proof or confirmation by biopsy or CMR is encouraged, and patient safety and CMR capabilities should also be taken into consideration.
Cardiovascular Injury
Injury and death of cardiomyocytes and infiltration of the tissue with immune cells are the hallmarks of acute myocarditis. CVB3 protease 2A induces cardiomyocyte apoptosis and cleaves dystrophin, which contribute to the development of cardiomyopathy.8 As inflammation subsides, the heart can recover. In some patients, the inflammation progresses to ventricular remodeling. Viral persistence may contribute to progression because the detection of enterovirus RNA portends a worse prognosis, whereas a polymorphism in the CC chemokine receptor-5 associated with enterovirus clearance reduces mortality.9
Causes
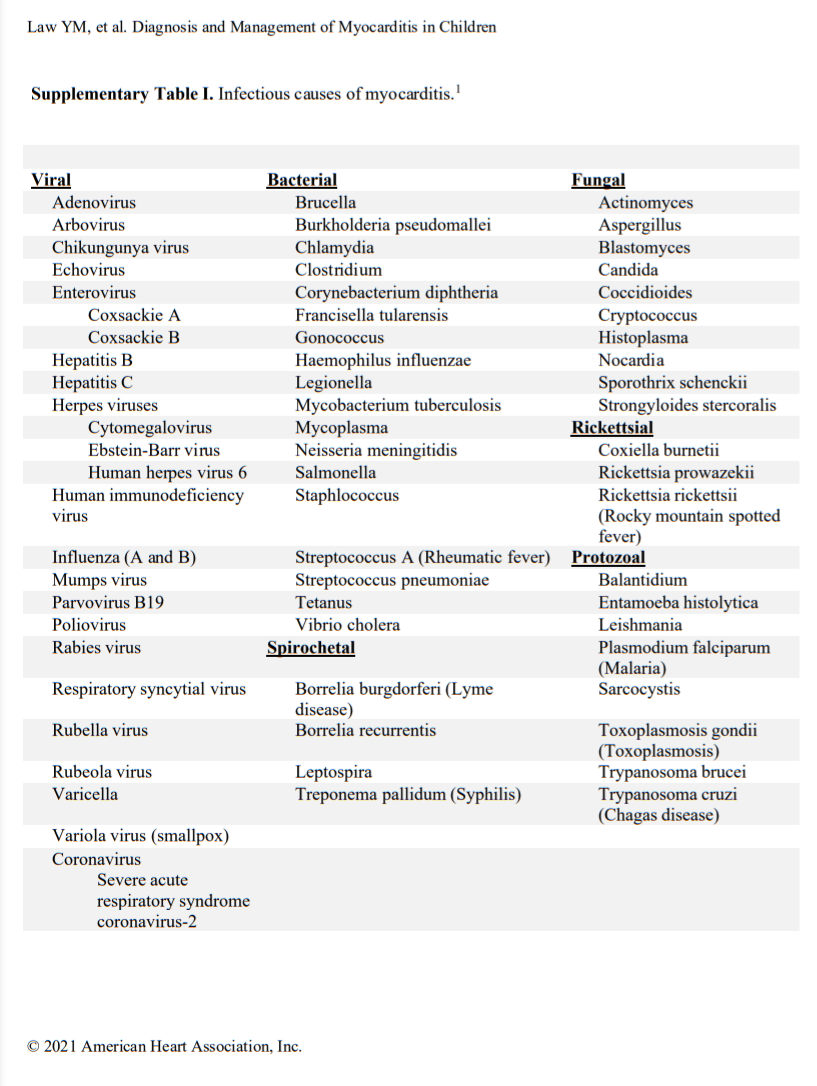
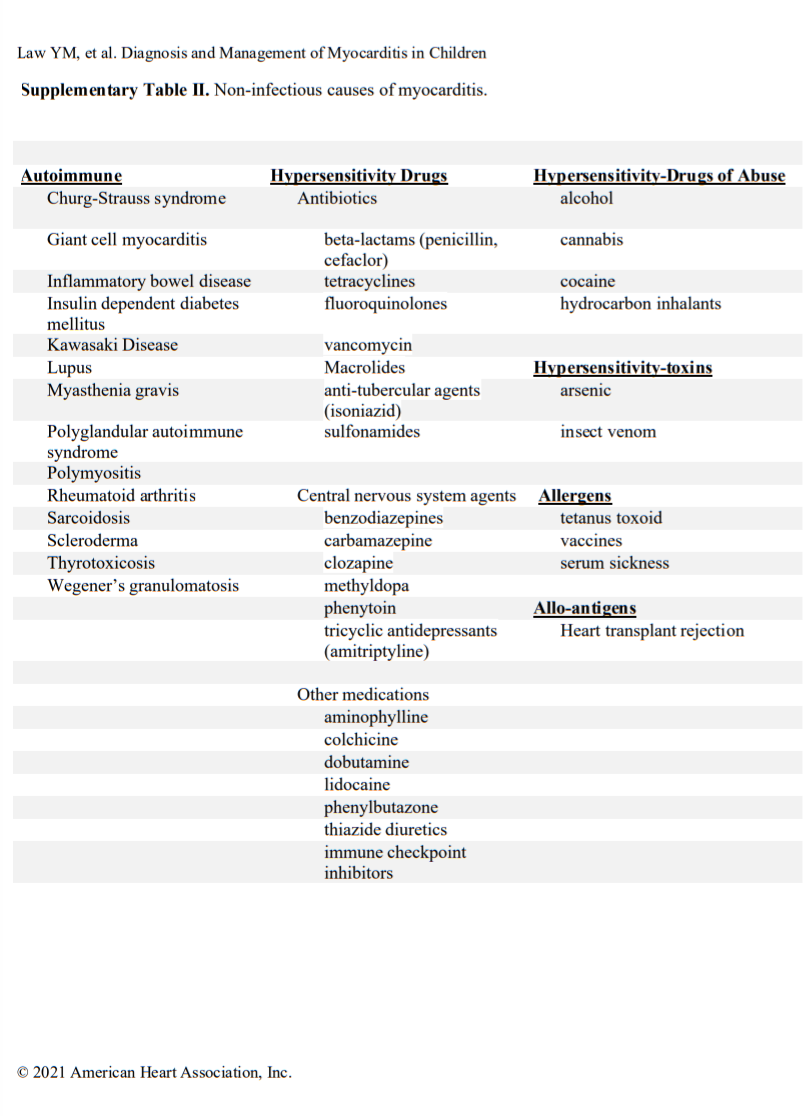
Acute myocarditis commonly has an infectious cause (Table I in the Data Supplement), with viral causes being the most prevalent. Specific viral causes have been shown by polymerase chain reaction (PCR) analysis of myocardial tissue and have demonstrated that the prevalence of specific viruses has shifted over time from predominantly adenovirus and enteroviruses to parvovirus B19 and human herpesvirus 6.10,11 Despite this shift, a diverse array of infectious (Table I in the Data Supplement) and noninfectious (Table II in the Data Supplement) causes of myocarditis remains. More recently, during the coronavirus disease-19 (COVID) pandemic, a novel form of shock with ventricular dysfunction emerged in children and is attributed to severe acute respiratory syndrome coronavirus 2.12,13 It is called multisystem inflammatory syndrome in children and likely involves inflammation of the heart and vasculature during or after the active infectious phase. Signs of myocardial inflammation and viral nucleic acid have been observed in autopsy cases.14,15
Clinical Manifestation and Diagnosis
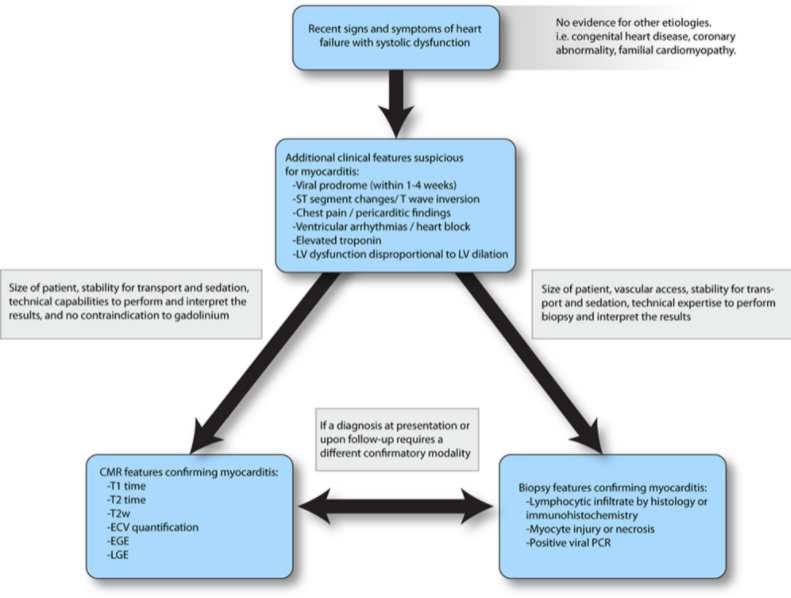
Myocarditis presents as several distinct clinical profiles. Typically, acute myocarditis presents with a poorly functioning ventricle with or without dilation, recent heart failure symptoms, and viral infectious symptoms in the preceding weeks.20,21 Fulminant myocarditis presents as cardiogenic shock; tachyarrhythmias are common, and inotropic or mechanical circulatory support (MCS) may be needed.22 Chronic persistent myocarditis occurs in the setting of cardiac symptoms such as chest pain, often with preserved systolic function, and histologic evidence of persistent myocardial inflammation.23 There have also been reports of pediatric patients with recurrent myocarditis, defined as episodes of acute myocarditis with intervals of clinical resolution.24Figure 3 provides a simplified diagnostic pathway for myocarditis. A comprehensive clinical assessment is critical because it places the presentation in a clinically suspected tier for myocarditis from which additional medical decisions on advanced diagnostics and therapy are to be made.
Figure 3. A simplified framework to confirm the suspicion of myocarditis. This diagnostic approach leverages various modalities, starting with clinical features followed by the application of cardiac magnetic resonance (CMR) or endomyocardial biopsy to confirm clinically suspected myocarditis. ECV indicates extracellular volume; EGE, early gadolinium enhancement; LGE, late gadolinium enhancement; LV, left ventricular; PCR, polymerase chain reaction; and T2w, T2-weighted imaging.
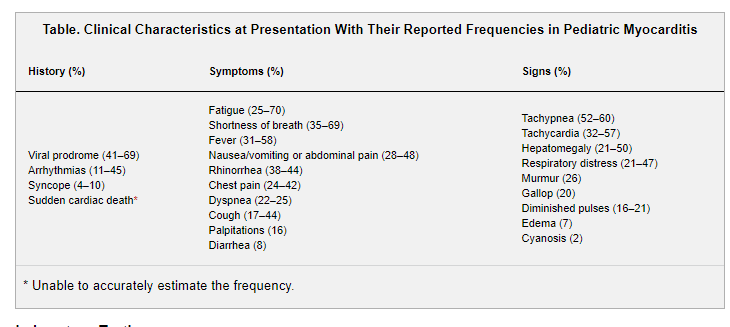
Signs and Symptoms
Presenting signs and symptoms (Table) have been reported in several large (n>150) pediatric series.2,21,25 History of a preceding viral prodrome is present in about two-thirds of patients. Fever has been reported in >50% of patients at presentation in a single-center pediatric study comparing biopsy-confirmed myocarditis with DCM (58% versus 15%; P=0.002); other cardiovascular complaints were not different between groups.26 Arrhythmias occur in up to 45% and include ventricular and atrial arrhythmias and high-grade atrioventricular block.21,27 Syncope occurs in ≈10%.21 Myocarditis can also present with sudden cardiac death and has been identified in 6% of young athletes with sudden cardiac death in the United States.28
Laboratory Testing
The use of serologic markers in cardiovascular disease is common and may assist with diagnosis, identify ongoing myocardial damage, and offer insight into prognosis.11,29,30 However, available biomarkers lack specificity, and no biomarker can differentiate myocarditis from other causes of acute myocardial dysfunction, injury, or ischemia.31 Traditional markers of cardiomyocyte lysis, including creatine kinase MB and troponins, may be elevated in acute myocarditis and are readily available. Troponin I and troponin T are more commonly used in pediatrics and can be elevated (troponin leak) in children with acute myocarditis. Troponin does not appear to be a sensitive or specific enough marker of biopsy-proven myocarditis. When elevated, it is often detected at very high levels.30 Troponin elevation has not been correlated with cardiac dysfunction or arrhythmias, but higher troponin levels have been associated with the use of extracorporeal membrane oxygenation (ECMO) and mortality.2,27 BNP (B-type natriuretic peptide) and NT-proBNP (N-terminal pro-BNP) are commonly elevated at the time of presentation and are associated with cardiac dysfunction, signs of acute heart failure, and the need for cardiopulmonary resuscitation or MCS.2 However, natriuretic peptides are generally related to heart failure, not specifically to myocarditis. Trending these biomarkers may be more useful than analyzing a single random sample other than at the time of presentation to screen for a cardiac problem. Nonspecific serum markers of inflammation, including leukocyte count, sedimentation rate, and C-reactive protein, can be elevated in cases of acute myocarditis or systemic inflammatory disorder, but normal values do not exclude an acute myocardial inflammatory process.20
Immunohistochemistry and viral genome analysis of the myocardium by PCR can characterize immune cells and specific pathogens (see also the Histopathology section).
PCR is extremely sensitive and specific and may identify the viral genome in the heart in ≈45% to 50% of suspected cases.10,34 It can also define viral load qualitatively and has been shown to correlate with outcome in some studies. PCR can identify viral genome in peripheral blood, stool, and respiratory secretions of patients with myocarditis in about one-third of cases and is often used as a surrogate of tissue PCR to make a presumed diagnosis. However, it should be noted that the correlation of peripheral samples with disease is poor and should not be substituted for myocardial viral PCR unless it obtaining an endomyocardial biopsy is infeasible.35 PCR is readily available for the most common viruses involved in myocarditis such as parvovirus B19, adenovirus, enteroviruses, Epstein-Barr, cytomegalovirus, and herpesvirus type-6.
Electrocardiography
Electrocardiographic features in children are variable and include sinus tachycardia, nonspecific ST-T–wave changes, T-wave inversion, ST-segment elevation, low-voltage QRS complexes in the limb leads, and atrioventricular conduction delays.36 A pattern of myocardial injury or infarction may be seen with the ST-segment changes and may be diffuse or in a defined coronary distribution pattern. With time and myocardial damage, pathologic Q waves may be seen and were described specifically with parvovirus B19 myocarditis by PCR in 1 study.37 Pericarditis may also be present demonstrated by ST-segment elevation and PR depression. Atrioventricular block, ventricular tachycardia and fibrillation, supraventricular tachycardias, and atrial fibrillation or flutter may occur and can be the presenting sign.38 Myocarditis should always be ruled out in a patient with new-onset third-degree heart block.20
Echocardiography
Echocardiography is the first-line and most widely used imaging modality for the evaluation of cardiac structure and function in patients suspected of myocarditis. Real-time imaging and portability are key strengths for quickly assessing the severity of cardiovascular compromise, especially in children who may have limited cooperativity or hemodynamic instability. Echocardiography reliably demonstrates the variable findings associated with myocarditis, including the following39:
Subtle to profound changes in global left ventricular (LV) or right ventricular systolic function, including regional wall motion abnormalities
Variable degrees of LV enlargement
Thickened myocardium from wall edema
Pericardial effusion
Intracardiac thrombus
Functional valvar regurgitation
Although generalized, a set of markers supportive of myocarditis would be the severity of LV systolic dysfunction and increased wall thickness that are disproportionate to the degree of LV dilation. Although it is the mainstay of diagnosis and surveillance, there are limitations to echocardiography in differentiating myocarditis from other pathogeneses. Tissue Doppler and myocardial strain can detect subtler changes in systolic or diastolic function, which may correlate with tissue pathology observed on biopsy or CMR.40–42 LV end-diastolic dimension43 and severity of dysfunction2 are reported to be associated with outcomes.
CMR Imaging
Although a common indication for performing CMR in adults is to distinguish myocarditis from coronary ischemia, the main objectives in children are to identify myocardial injury and to detect inflammatory features to distinguish acute myocarditis from noninflammatory cardiomyopathies.
Given the heterogeneous and often nonspecific presentation and echocardiographic findings of myocarditis,20 more sensitive and specific imaging techniques are needed.22 CMR can demonstrate markers of inflammation and necrosis that characterize myocarditis histologically. Beyond tissue characterization, CMR is the gold standard for quantification of ventricular volumes, ejection fraction, and mass.
Clinical markers of inflammation by CMR include high signal intensity on T2-weighted imaging (Figure 4A), increased T2 (Figure 4B) and T1 (Figure 4C) times denoting extracellular volume (ECV) fraction, myocardial thickening attributable to edema, and rapid uptake (early gadolinium enhancement) of contrast as a sign of hyperemia.44 In addition to edema, T1 and ECV are markers of diffuse myocardial fibrosis in chronic disease processes such as cardiomyopathies. Measures of necrosis include contrast retention 10 to 15 minutes after injection of gadolinium, indicating either acute myocardial injury or scar formation (Figure 4D).
Treatment
Acute myocarditis in children can progress rapidly to hemodynamic compromise, sometimes even in the setting of borderline systolic function and especially with the emergence of arrhythmias.52,53 Anticipatory care is necessary. Judicious triaging of the disposition of patients seen in ambulatory or emergency care locations for workup or for management of suspected myocarditis is crucial. It is also important to determine whether monitoring in the intensive care unit is necessary. The ability to closely monitor the cardiovascular status, including the rhythm continuously, should be considered. If the trajectory is toward hemodynamic compromise, consideration should be given to transfer to a center that provides pediatric MCS and transplantation.
A critical aspect in the early phase is monitoring for atrial or ventricular arrhythmias. Arrhythmias may be associated with a poor early outcome.27 The management of arrhythmias is outside the scope of this statement and is covered elsewhere54; however, it should be noted that there are no specific antiarrhythmic medical therapies related to myocarditis. It is unclear whether treatment of myocarditis lessens the development of arrhythmias. Bradyarrhythmias and heart block are rare presentations. Temporary transvenous pacing should be considered because the rhythm disturbance may be transient but hemodynamically compromising with progression of ventricular dysfunction.55 Cardiac monitoring in an inpatient setting is reasonable if myocarditis is suspected.
Supportive care is similar to other scenarios of acute heart failure. Low cardiac output should be treated promptly. Milrinone typically is used as first-line therapy for inotropy, whereas inotropes with vasopressor properties such as epinephrine and dopamine are generally reserved for those with hypotension and cardiogenic shock because these agents have more chronotropic and arrhythmogenic potential.2 Calcium chloride and vasopressin infusion can also augment perfusion pressure without adding to cardiac stimulation. Oral heart failure therapy should be initiated once the patient is beyond the acute stage of illness and shows persistent systolic dysfunction or heart failure. Because acute myocarditis leads to myocardial injury, similar to acute myocardial infarction in which reverse remodeling drugs are used even without LV systolic dysfunction, it is unclear whether the same protective approach should be taken in children with myocarditis whose LV function has normalized (see the section An Adult Perspective on Pediatric Myocarditis).
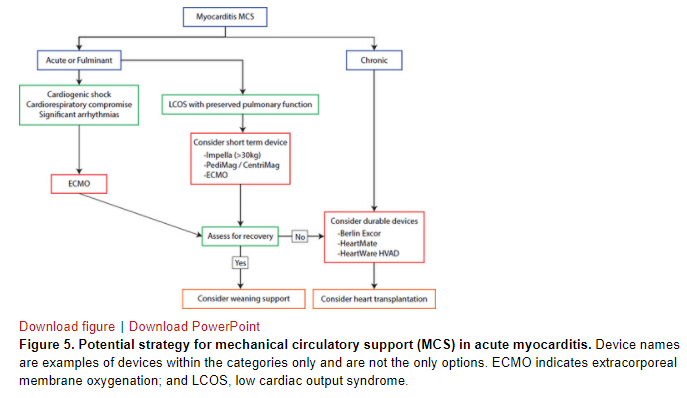
Early intervention with MCS should be considered and can be lifesaving. Thus, patients should be cared for at a pediatric center with expertise in MCS and transplantation. Registry data show that 23% of patients during the early phase of hospital admission require MCS with either ECMO or a ventricular assist device (VAD).1 The suggested algorithm for type of MCS based on the clinical scenario is shown in Figure 5. ECMO is an attractive choice of MCS because it can be deployed emergently and because the probability of a brisk cardiac recovery in myocarditis is high compared with cardiomyopathy. Alternatively, percutaneous support with Impella has been reported with reasonable success and can be deployed expeditiously to support the LV with unloading.56 If ECMO cannot be weaned, consideration is given to transition to durable VAD as a bridge to recovery or transplantation (Figure 5). There are no data to compare the superiority of the use of VAD preemptively (electively) or of VAD over ECMO.
Regardless of the choice of MCS, early left-sided heart decompression is important in patients with severe LV dysfunction or stunning to maximize the opportunity for recovery. A percutaneous atrial septostomy, surgical placement of a vent, or use of Impella accomplishes LV unloading. ECMO support has a 69% to 76% survival to discharge rate. Up to 24% of patients on Berlin Excor may be weaned off, and patients on VAD should be assessed serially for ventricular recovery.20 Patients who require long-term MCS or who recover from the acute episode but develop severe chronic heart failure may require heart transplantation.57